The new study shows that a superfood-based food supplement had dramatic results in prostate cancer patients. And yet, with exquisite irony, the term ‘superfood’ is facing a possible European Union (EU) ban.
Pomi-T® and prostate cancer
The UK-based Pomi-T® study, funded by the National Health Service (NHS) National Cancer Research Network (NCRN), involved a total of 203 men aged 53–89 years with histologically confirmed prostate cancer. The patients had previously undergone radical prostatectomy or surgical removal of the prostate and surrounding tissue, and were being managed by active surveillance or watchful waiting. They were randomised to 6 months of treatment with either a placebo or a twice-daily dosage of Pomi-T®, a naturally derived food supplement containing pomegranate seed, green tea, broccoli and turmeric.

Promising study results
The results were impressive. Six months of treatment with the supplement led to significantly slower increases in prostate-specific antigen (PSA), compared with placebo treatment (difference of 63.8%, p=0.0008). Furthermore, PSA levels rose by a median of 14.7% in the Pomi-T® group, compared with a median rise of 78.5% for patients in the placebo group (Figure 1).
Significantly more men in the Pomi-T® group (n=61 [46%]) had a stable or lower PSA at the end of the trial compared with men in the placebo group (n=9 [14%]; p=0.000010). Significantly more men in the Pomi-T® group (n=114 [93%]) were still managed through active surveillance or watchful waiting by the end of the 6 months, compared with placebo-treated men (n=38 [74%]; p=0.01).
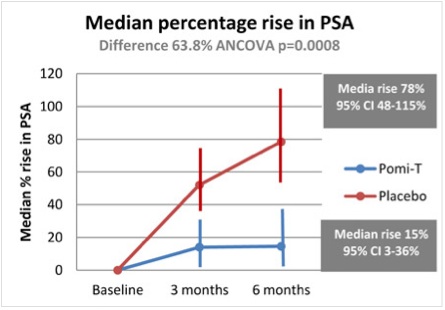
Figure 1. Median percentage PSA rise in men with prostate cancer in the POMI-T study. Taken from Thomas R et al. J Clin Oncol 2013;31 (Suppl; abs 5008).
Nature's antioxidant superfoods vs. EU health claims madness
So, promising results indeed for Pomi-T® in prostate cancer – and perhaps unsurprising, since pomegranate seed, green tea, broccoli and turmeric all contain high levels of polyphenols and other phytochemicals, which are both antioxidant and anti-inflammatory. Since inflammation plays an integral role in cancer development, anti-inflammatory strategies make sense. Antioxidants, too, have a biologically rational role to play in cancer prevention and treatment, despite recent controversy.
Unfortunately, the EU doesn’t see it that way. One of the more disturbing consequences of its Nutrition and Health Claims Regulation (NHCR; No. 1924/2006) is that not a single antioxidant-related health claim has been approved by the European Food Safety Authority (EFSA). Even though Pomi-T® has a randomised, controlled trial in its favour, it won’t be eligible because it was performed using prostate cancer patients, rather than healthy subjects.
Jettisoning ‘generic descriptors’
And if the European Commission decides to ban ‘generic descriptors’ that imply the product bearing them has a health benefit, we can wave goodbye entirely to phrases like ‘superfood’. Terms like ‘antioxidant’ would only be applicable for individual foods, ingredients or products for which elevated antioxidant activity can be shown in healthy subjects following clinical trials. So far, it seems no-one has been able to justify the cost of such trials. This leaves products like Pomi-T® voiceless and the public unable to learn about its scientifically proven benefits.
Irrespective of how food-like a product is, the only option that allows a manufacturer to make a health benefit claim that relates to a diseased population is to have the product licensed as a drug. This a privilege only the biggest companies can afford – and provides a demonstration of just how far the EU has strayed from Hippocrates’ advice to, “Let food be thy medicine and medicine be thy food”.








Comments
your voice counts
12 June 2013 at 9:32 pm
Yep, world gone mad, insidious & under the Corporate lobby influenced legislative mechanisms pushing a globalist agenda. Love your work there at A.N.H & Bob. Met you Bob at the Cancer Conference event at Totnes where you spoke. I am heavily motivated to protest against ehuman state of affairs after attending the Bilderburg Fringe counter festival event at Watford last weekend. Alex Jones of infowars.com would have something to say on this!
13 June 2013 at 1:07 am
This is a very small study to be statistically significant IMHO. Also, I understood that the PSA count is of little to no value. Am I wrong or is this very misleading?
13 June 2013 at 11:16 am
Hi Steve, thanks for your comment. While this was indeed a fairly small trial, that in no way precludes it from being statistically significant, and the results are far too promising to dismiss merely on the basis of sample size. There is an ongoing trend, bordering on an obsession, for larger and larger sample sizes in clinical trials. It’s worth bearing in mind that if significance between treatment and controls is demonstrated in a small sample size, the results would likely show even greater significance if the samples sizes were large, owing to the reduced variance in means.
As for PSA, we agree that it's controversial. One of the links we provided was to an NHS leaflet explaining why PSA-based prostate cancer screening isn't offered on the NHS: http://www.cancerscreening.nhs.uk/prostate/prostate-patient-info-sheet.pdf. That said, PSA testing is still offered to men by the NHS and is widely used both in a clinical setting and in research: http://www.ncbi.nlm.nih.gov/pubmed/?term=psa. And don't forget that fewer men in the Pomi-T group demonstrated clinical progression of their disease, as shown by a change in management - a result entirely independent of PSA.
13 June 2013 at 2:37 am
I think Alliance For Natural Health needs to get up to speed on 'alternatives' for cancer. I hope I can help readers/members/editors etc. with my over half a century in the Alternative Health Industry. I have commented much on Naturalnews.com, Mercola.com, and in many papers, Telegraph, Express etc. every time one of their their 'writers' spins out some snippet of hope from Cancer Inc.
Let's start with...Dr Robert Good, the leader in immunology carried out the first bone marrow transplant in the mid 1980s in US. By then he had published over 2000 medical papers and had been nominated for The Nobel Prize 3 times. Dr. Good found the dietary, enzymatic cure for pancreatic cancer BUT he was refused publication in ALL med journals. Why? The Gate Keepers of Cancer Inc. protect their industry by controlling the now-exposed-as-corrupt* med.journals. Unpublished=unrecognised=illegal to apply.
*www.naturalnews.com/023074_ghostwriting_drug_trials.html.
We have Dr H R Clark, an ex Canadian Govt, cell research biologist who carried out over half a million repeatable, so scientifically valid bio-resonance tests. Dr Clark has precisely identified the specific parasites, bacteria, viruses, moulds, heavy metals, dyes,radio-active compounds, benzene, asbestos, food allergens etc. and their pathways, that cause all cancers. Dr Clark needed to publish privately, and so her work remains 'unrecognised' and 'illegal'.
Other alternative cures exist. These interrupt the cancer pathways as laid out by Dr Clark. They are from Drs. Raymond Rife, and Robert Beck; (electrical resonance); Dr Burzynski (gene targetting with drugs); Germany`s ozone protocol that cured Pres. Reagan's lung cancer; and the herbals of of essiac, that cured Sen Ted Kennedy's son's terminal bone cancer; and the herbals of Dr. Marijah McCain, H Hoxey and Jason Winters.
According to Dr Clark, the prostate is inflamed by two allergens, acetic acid, vinegar, and the phenol naringenin found in most citrus and highest in tangerenes. According to Dr. Clark, any inflamed body part allows easy entry for the cancer nucleus and cancer complex to start and fuel a malignancy at that site, in the presnce of nickel and a few other 'players'. As a male, my vinegar and citrus intake is Z E R O, and I ensure no nickel, ie cook only with 18/20 marked stainless steel, watch back taped etc.
13 June 2013 at 9:31 am
Any reason why ANH censored my comment on cancer, when a similar comment of mine was today published in the Telegraph?
http://www.telegraph.co.uk/health/healthnews/10114685/Cancer-database-could-save-thousands-of-lives.html
13 June 2013 at 10:53 am
Hi Thomas, we didn't censor anything. All comments are subject to moderation and yours was submitted while we were out of the office.
13 June 2013 at 3:16 pm
Why is my comment censored? Are you part of Cancer Inc, or is my comment too hot to handle?
13 June 2013 at 3:50 pm
Hi Thomas, we published your comment this morning and responded, as you'll see if you look further down the page.
You might also be interested in some of our other recent articles on cancer: http://anhinternational.org/Medicine_mutilation_or_stock_manipulation_The_strange_case_of_Angelina_Jolie, http://anhinternational.org/news/trials-of-the-totnes-cancer-health-care-conference, http://anhinternational.org/news/anh-book-review-the-cancer-survivor%E2%80%99s-bible-by-jonathan-chamberlain, http://anhinternational.org/news/how-maverick-cancer-treatments-are-suppressed-by-the-mainstream. For more, just put 'cancer' into the Search box at the top-right of the page. We're certainly not part of 'Cancer Inc'!
26 June 2013 at 11:23 am
In my opinion its all about healthy eating to avoid cancers. If u eat processed foods filled with chemicals then you're just putting yourself at risk. Especially men who need to maintain a health prostate.
Great Article!
11 August 2013 at 8:18 pm
Hello.Can you tell me where I can buy Pomi-T tablets from UK please?thank you
12 August 2013 at 3:19 pm
Hi there, just follow this link: http://www.pomi-t.com/ and click on the 'Order POMI-T' tab at the top of the page.
02 November 2013 at 10:25 pm
This article is EXTREMELY misleading. There is nowhere near a perfect correlation between PSA and prostate cancer, and on top of that the paper doesn't address whether or not it pomi-t was "masking" the PSA from being detected. DO YOUR OWN RESEARCH AND ONLY TRUST PEER REVIEWED LITERATURE….NOT A WEBSITE THAT MY LITTLE KID CAN MAKE.
Nowhere in the paper was there any mention of "benefiting prostate cancer patients" which further shows that whoever wrote this article is a complete moron and cannot think critically.
04 November 2013 at 2:16 pm
Two things, Anonymous. One, the article was based on an abstract published at the American Society of Clinical Oncology (ASCO) meeting on 2nd June 2013 – which is obvious from clicking the link we provided: http://www.pomi-t.com/asco-pomi-t-study-abstract/. If you’d prefer a link to the ASCO website, here it is: http://meetinglibrary.asco.org/content/112921-132. The abstract described preliminary results of a placebo-controlled, randomised, double-blind study organised by the UK National Cancer Research Network (NCRN) – http://ncrndev.org.uk/index.php?option=com_content&task=view&id=13&Itemid=46 – which was “established by the Department of Health in April 2001 to provide the NHS with an infrastructure to support prospective trials of cancer treatments and other well-designed studies”. In other words, this was an early report of a well-designed study funded by the UK National Health Service and run by experienced researchers working at UK hospitals. It’s about as mainstream as they come.
Two, we’re aware of the debate around the link between PSA readings and prostate cancers: http://anhinternational.org/news/anh-intl-feature-documentary-on-prostate-cancer-essential-viewing-for-all-men-over-40. We imagine that the authors of the POMI-T study are, too. Until the full study report is published, we have no idea whether or not the protocol addressed the aspects you mention. However, we do know that, “Significantly more men in the Pomi-T® group...were still managed through active surveillance or watchful waiting by the end of the 6 months, compared with placebo-treated men”. We’d say that significantly increasing the chances of not requiring drugs or surgery translates to great benefit for prostate cancer patients – wouldn’t you?
21 August 2014 at 3:36 pm
Nice article! Pomi-t saved my dad life! I am very happy with this!
13 April 2024 at 5:18 am
In Australia, I found it very difficult and very expensive to source Pomi T for my husband in 2015. Our Naturopath at the time suggested that the dose we used was too inadequate to be effective although he agreed it was a fine product. The only subsequent treatments needed were due to medical interventions, not disease progression so I'm assuming that the product was effective (in addition to our real food organic practices)
Your voice counts
We welcome your comments and are very interested in your point of view, but we ask that you keep them relevant to the article, that they be civil and without commercial links. All comments are moderated prior to being published. We reserve the right to edit or not publish comments that we consider abusive or offensive.
There is extra content here from a third party provider. You will be unable to see this content unless you agree to allow Content Cookies. Cookie Preferences