Content Sections
- ● Transcript
- 1. The vaccine: what and how
- 2. Risks from SARS-CoV-2
- 3. Vaccination risks and benefits
- 4. Testing prior to vaccination
- 1. Sufficient information for properly informed consent
- 2. Stop governments claiming covid vaccines re safe
- 3. Equal rights for vaccinated and unvaccinated
- ● Other petitions
With the UK being the first drug regulator in the world to greenlight a covid vaccine, we felt it important to get some important information out to you that cuts through the flannel that's being delivered via the mainstream media.
We haven't found we agree with a lot of commentary by Dr Tony Fauci, Director of the US National Institute of Allergy and Infectious Diseases (NIAID). But we concurred with him over his criticism of the premature rubber stamping of the BioNTech Pfizer vaccine this week that Fauci described as "really rushed".
“But they just took the data from the Pfizer company. And instead of scrutinizing it really, really carefully, they said, OK, let’s approve it. That’s it.’ And they went with it.”
- Dr Anthony Fauci, NAID, quoted by Politico
Apart from rushing the approval, there are a gamut of other problems with the decision to green light the trial (see study design):
- Many of the trial endpoints that relate to safety and efficacy are not yet complete, so it is impossible to draw conclusions about safety or efficacy until this is complete
- These include measuring covid-19 incidence per 1000 person-years (e.g. 1000 people for 1 year, 500 for 2 years, etc) of follow-up following vaccination (that should include reactions after naturally-acquiring infection to evaluate any post-infection vaccine-associated hypersensitivity)
- Immunogenicity (in terms of S1-binding IgG levels and/or BD-binding IgG levels, and SARS-CoV-2 neutralising titers) will be measured up to a year after the second dose has been delivered (and the MHRA is unlikely to have seen more than 2 months worth of data)
- None of the data sets have been released for scrutiny by independent experts
- Immunodeficient patients were excluded yet will be vaccinated
- Cases were all confirmed using one of three RT-PCR tests (Cepheid Xpert Xpress, Roche comas SARS-CoV-2 RT-PCR, Abbott/RT SARS-CoV-2 assay) that are flawed as a diagnostic method for determining transmissible SARS-CoV-2 infection
- Pfizer has reported 3.8% severe (Grade 3) adverse events despite claiming "no serious safety concerns". This is misleading given that the vaccine industry and regulators use the term "serious" only in relation to reactions causing hospitalisation or death (grade 4 and 5 adverse events, respectively). In the Phase 3 trial, up to 3.8% of test subjects suffered severe (Grade 3) adverse reactions (severe headache, undoubtedly caused by a severe, systemic inflammatory reaction). If that percentage was applied to 70% of the US and UK populations, that would amount to a staggering 10 million+ people who would experience severe adverse events
- The trial design and results have not been subject to external peer review
- The UK government has taken the "precautionary step" to add the emergency authorised covid-19 vaccine to the Vaccine Damage Payments Scheme that limits payments to £120,000 for proven vaccine damage.
- Pfizer has legal protection from the UK government in the event of injury to those who are vaccinated.
In the patient information leaflet, "Very common side effects that may affect more than 1 in 10 people" (some of which overlap with covid disease symptoms) are listed as:
- pain at injection site
- tiredness
- headache
- muscle pain
- chills
- joint pain
- fever
We have produced the following video (see transcript with links below) to emphasise that, at the time of writing, there is not currently sufficient information to allow any member of the public to give properly informed consent for the BioNTech/Pfizer or any other covid vaccine. That doesn't mean that some may wish to be vaccinated regardless and give their consent in the absence of more complete information. Assuming you reside in a country that has not mandated the vaccine, you have the right to refuse or delay the vaccine, pending provision of further information.
IMPORTANT NOTE: Since the video was recorded on Tuesday, the BioNTech/Pfizer vaccine composition, including the nanoparticle composition, has been released by the MHRA but it does not include concentrations of ingredients making it impossible to assess toxicology. The ingredients will include 30 micrograms mRNA in each dose, along with:
- ALC-0315 = (4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate),
- ALC-0159 = 2[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide,
- 1,2-Distearoyl-sn-glycero-3-phosphocholine,
- cholesterol,
- potassium chloride,
- potassium dihydrogen phosphate,
- sodium chloride,
- disodium hydrogen phosphate dihydrate,
- sucrose.
The ALC-0315 is a hexane containing compound and these are known to be potentially neurotoxic. ALC-0159 contains polyethylene glycol (PEG) that is associated with hypersensitivity and allergenic reactions. The toxicological profile of the mRNA delivery system cannot be determined because neither have the concentrations been declared, nor has the nanoparticle delivery system, surface charges and other physicochemical characteristics been declared. These may dramatically increase the toxicological profile.
Transcript
Hi there, my name’s Rob Verkerk – and welcome to our latest coronacast. With the news that the UK regulator, the MHRA, has this week given the green light for emergency authorisation of the BioNTech/Pfizer vaccine, we’re obviously going to talk vaccines in this week’s coronacast. For those of you who’ve followed our campaign to pressurise governments and vaccine makers to be transparent on their science, it won’t be a surprise to you to find the roll-out that might start as early as next week will occur in the absence of key information that’s really needed for people to be able exercise properly informed consent. So we’re going to drill down into some of the key pieces of information that will hopefully help shine a little more light into the opaque box of vaccine information.
As more vaccine doses are received, and more vaccines receive authorisations, much of the world’s population will soon be asked to make a momentous choice: vaccinate using the first ever fast-tracked synthetic biology vaccines. Or not, as the case may be, and then face the possible withdrawal of basic rights or privileges. To help you make this choice you need access to specific pieces of information that allow you to give medical informed consent that, as explained in a previous video, is a legal obligation on the part of healthcare providers and authorities.
This video has been made to help you understand what’s known or not known about four key pieces of information you have a right to know before making this choice:
The first is knowing what the vaccine is, including what’s in it, and how it works. This is important because this is the first time synthetic biology vaccines have been released at scale.
The second is having a clear understanding of what you’re protecting yourself from, and that relates to the current not past health risks posed by the virus that causes covid-19 disease, namely SARS-CoV-2, at the time you need to make an informed decision about vaccination.
The third is knowing about what benefits and risks the vaccine might offer you in the wake of news of claims of 62% to 95% efficacy among the covid vaccine front-runners from BioNTech & Pfizer that we’ll refer to as just Pfizer in thiss video, Moderna and Oxford University & AstraZeneca, that we’ll just refer to as AstraZeneca.
The fourth and final area is knowing what information you can get about your immunity status before being tested before you decide to vaccinate.
These are all components of the 10-point vaccine transparency manifesto that we launched last May.
1. The vaccine: what and how
Let’s start by looking at how the UK grown AstraZeneca vaccine works.
Source: Covid-19 Vaccine Trial
This vaccine is called a Non-Replicating Viral Vector vaccine. It’s the same technology platform that was used for MERS and zika vaccines as well as some flu vaccines. It uses a vector or transporter that’s a common cold virus that infects chimpanzees that’s been genetically altered so it can’t replicate in humans. Into this GM chimp virus is inserted a piece of synthetic genetic material that codes for the surface spike protein of SARS-Cov-2. Once it’s injected into a human being, the surface spike protein is expressed from the synthetic gene sequence and an antibody response to the antigen is produced, this providing protection from a trained immune system if you then encounter SARS-CoV-2 during the yet to be understood time period in which the immune protection has been enhanced. This vaccine can be distributed and stored at normal refrigerator temperatures of between 4 and 8 degrees Celsius. It’s reckoned to cost around £3 sterling per dose and it’s the 2 dose schedule that was found to given 90% efficacy under trial conditions, one dose offering just 62% by comparison.
Both the Pfizer and Moderna vaccines use a different vaccine platform – one called messenger RNA or mRNA for short. Messenger RNA itself is a single strand of the nucleic acid RNA that corresponds to a particular genetic sequence that codes for a given gene, that is in turn read by a ribosome within the cell to synthesise the specific protein that is normally produced by that gene. mRNA vaccines use a synthetic gene sequence that codes for the spike protein of SARS-CoV-2. They are encapsulated within miniscule lipid nanoparticles that when injected into muscle, causes the muscle cells to start producing the spike protein. This then causes the body to mount an immune response which should also protect someone who is infected with the real virus during the unknown period of time in which the immune system is primed. By giving the instructions to the body to produce the spike protein, mRNA viruses are in effect turning the body into the vaccine factory. The mRNA vaccines require 2 doses that cost between £15 and £25 sterling per dose and need to be stored at minus 70 degrees Celsius which complicates the cold distribution chain currently used for vaccines and certain drugs.
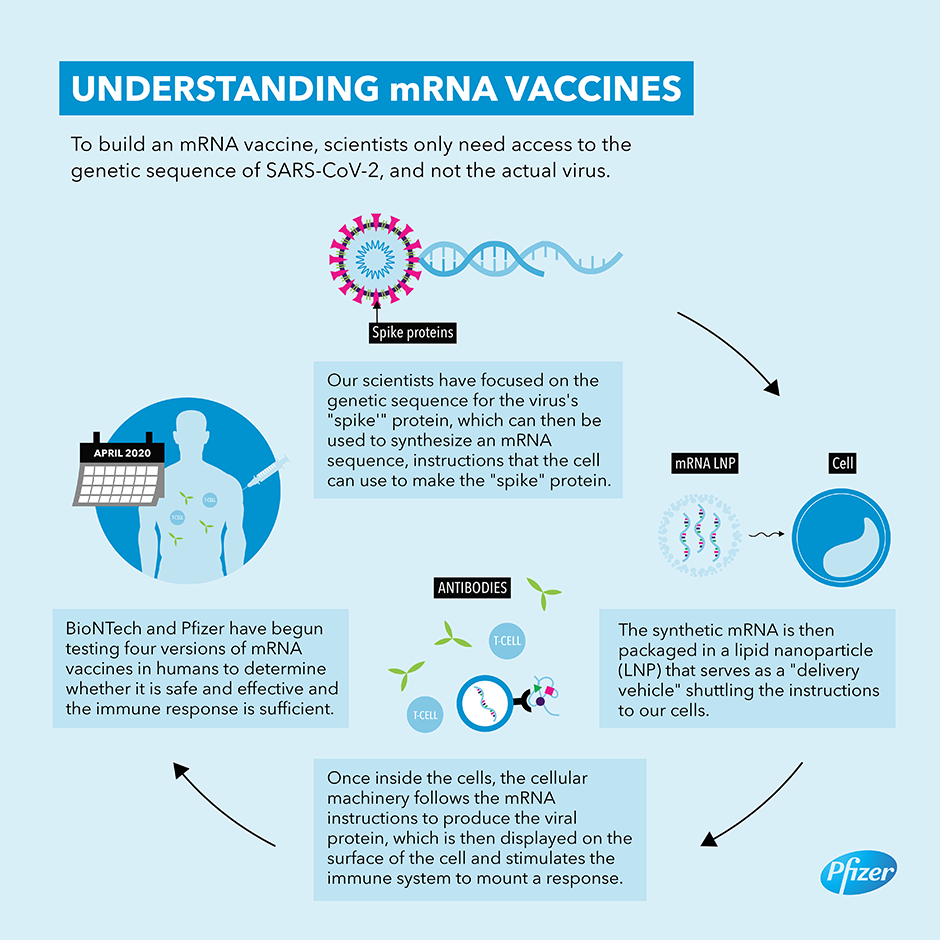
Presently, there isn’t any reliable information on exactly what the lipid nanoparticles in the mRNA vaccines are comprised of, whether there are any other ingredients or adjuvants added to any of the vaccines, or if there might be any potential contaminants in each of the main vaccine candidates.
Moving on to how the vaccines affect the immune system – the main, so-called primary, endpoints being evaluated in the Phase 3 trials for the current round of emergency use authorisation reviews are safety issues. Some are also evaluating covid symptoms among those who test positive by PCR, and others look at raised neutralising antibodies.
Some also include endpoints round safety that won’t complete until well after emergency use authorisation so in our view it is entirely disingenuous and unscientific for health authorities to make the claim that these vaccines are safe.
2. Risks from SARS-CoV-2
If you want to participate in the Phase 3 trial of the Oxford AstraZeneca vaccine you’re still in with a chance as it’s running a little behind the Pfizer and Moderna schedules. But you’ll only be prioritised if you’re at high risk of exposure, such as a frontline health worker or care home worker. The reason given by the Oxford researchers is that the epidemic is waning and they need to make sure enough people are exposed to the real virus to get enough data to see how vaccinated and control populations respond when they’re infected. You heard that – didn’t you? The university that’s ranked as the world’s number 1 since 2017 by The Times Higher Education World University Rankings says the epidemic is waning.
Last week, Dr Mike Yeadon, a former Vice President and Chief Science Officer for Allergy & Respiratory at Pfizer, along with others, presented a detailed briefing to UK Members of Parliament this week that is supporting what is described as a parliamentary rebellion – which I guess it is in some ways as it’s rebelling against groupthink and politics attempting to take over healthcare and driving a coach and horses through people’s rights and freedoms .
The briefing argues with supporting data that the pandemic is now over and what we’re really seeing now in the northern hemisphere is a pseudo-epidemic propagated by a flawed mass testing regime reliant on PCR that’s generating large numbers of false positives – enough to give the impression of an epidemic. False positive pseudo-epidemics are actually well known in the medical literature and have been found in everything from TB to prostate cancer to whooping cough, and have been on the rise with increasing reliance on single target PCR testing such as that used for SARS-CoV-2.
We’re also seeing a tendency for excess mortalities going up in countries that didn’t experience any first wave excess mortality. Some of this might be linked to delayed indirect effects of lockdowns when people with serious diseases haven’t been able to access the care they needed while some may be experiencing a delayed first wave.
3. Vaccination risks and benefits
It’s essential that all known risks, relating both to the pathogen but also to the particular vaccine in question, are put in the public domain, along with what’s known about the protection the vaccine offers. That’s not just headlines like 90 to 95% efficacy. That means putting the raw data into the public domain so it can be analysed by independent scientists. To-date, none of the full datasets have been released.
>>> Covid-19 vaccines: where are the data? in The BMJ, 27 November 2020
Not only that, none of the three frontline vaccines from AstraZeneca, Pfizer or Moderna have published their Phase 3 trial results. The only things we’ve got to go on so far are press releases that are deeply deficient in data on both risks and benefits. What has been blasted around the airwaves of course are these dizzying efficacy rates of beween 62 and 95% that past history from vaccine trials and post-marketing surveillance suggests are unlikely to be achieved in the real world.
So what do these 62 to 95% headlines really mean? First, they relate to efficacy, not effectiveness. Efficacy measures the performance of a treatment under ideal and controlled circumstances, while effectiveness is the performance under real-world conditions. Because the vaccine are being evaluated under trial conditions, you don’t have the vagaries of the real-world to contend with. And what are the performance parameters? Is it protecting people against transmitting the infection, or is it about protecting the vaccinated person from severe disease if you are infected during a specific time window.
>>> Call by Dr Peter Doshi to "be cautious and first see the full data" in The BMJ, 26 November 2020
As it turns out, it’s only the latter. That means vaccinations are not being evaluated for their ability to stop the transmission of infections – something you’d think would be a target if you wanted to wipe out an epidemic or pandemic. But it’s actually something that takes much longer than the very short time frame these vaccines are being created within. The same applies to safety issues – some of the trials will continue to look at safety issues for 12 months or more, but the vaccines will be rolled out at least for emergency use sometimes with just a couple of months of safety data.
And as we’ve said before, history tells us it can be years before safety concerns are exposed, as we discovered with the swine flu vaccine Pandemrix and narcolepsy in children.
The current crop of novel covid vaccines are only being tested for their ability to stop people getting seriously ill, a risk that becomes less and less in a waning epidemic, and a risk that primarily only affects older people or those with underlying conditions.
On top of that, we don’t know a lot about the populations who appear to be protected from severe disease, and how many of these include groups who are the most vulnerable. Also note that two doses are needed in all 3 vaccines to yield the highest immune response – and higher dosages generally also yield more adverse reactions.
In effect, it means that the current Phase 3 trials are really testing the vaccines as a preventative treatment, not for their ability to make you immune to the virus and incapable of transmitting it to others. This is an important distinction. In other words, what the vaccines are really trying to do it make more people respond just like an unvaccinated healthy person who has a good functioning immune system, with some possible historic cross-immunity to other coronaviruses or previous exposure to SARS-CoV-2, and adequate amounts of vitamin C, D, zinc and other cofactors in their system.
On the risks side, AstraZeneca, Pfizer and Moderna have all claimed a lack of safety concerns. But in the Phase 1 and 2 trials of the AstraZeneca vaccine, moderate to severe adverse events were experienced by 70% of those receiving the covid jab. With the Pfizer vaccine, nearly 4% of people suffered from severe – or Grade 3 – adverse events. These grade 3 adverse events aren’t a walk in the park – they’re just one category down from grade 4 adverse events that are described as “potentially life threatening events” that require hospitalisation and critical care.
People with Grade 5 events don’t get to tell their story, but the more separated in time a death is from a vaccination, as hundreds of families have found in the various national vaccine courts, the harder it gets to prove a causal relationship.
Anyway, Grade 3 adverse events are certainly severe enough to give a susceptible person sufficient immunologic or neurologic shock to trigger long-term health challenges that might affect their nervous system or autoimmunity. And while Pfizer might have dismissed these as insignificant at just 3.8%, if that percentage was applied to say 70% of the US and UK populations, that would amount to a staggering 10.6 million people who would experience severe adverse events. The trouble here is that the potential for long-term consequences of an adverse event isn’t something you can just monitor in a couple of months – typically it takes years of post-marketing surveillance. And here I want to emphasise the point: Until that kind of time scale has passed, we think it would be premature, irresponsible and unscientific to call these vaccines safe.
4. Testing prior to vaccination
What if you’ve already had Covid, knowingly or unknowingly? If you’ve had Covid quite severely and you’re being tested for your levels of antibodies – they may still be raised. But if you’ve had milder infection, or you already have some cross immunity from another related coronavirus, and in particular if you’re a healthy woman, you might not have raised antibodies at all. Yet you might still be immune to infection.
What would protect you is your memory T cells like CD4+ and CD8+ T cells, along with B cells, all of which aren’t measured when you have one of those “if I had it in the past” tests. These actually don’t accurately tell you if you had it in the past as they only measure one kind of marker – up to three different immunoglobulins, IgA, IgM or IgG - for longer-term, adaptive immune responses – namely neutralising antibodies. Not the all-important memory T cell response that is the main driver of herd immunity.
If immunity passports are to be developed which is the plan of the UK and many other governments, it would make no sense to do this without T cell tests being included. We know from other pathogens including the closely related SARS that memory T cells may remain active against a given pathogen for years, even decades. We also know that antibodies always fade after a few months.
This brings us on to what we can all do.
I know for many people, as long as the vaccines don’t become mandatory in their country or region, deciding whether or not you, your loved ones or your children should or shouldn’t have a covid vaccine isn’t going to be an easy decision. The first thing that needs stressing is that given the uncertainties around any medical intervention, the information you have at your disposal that forms of the basis of you exercising your right to consent or not is always going to be imperfect or incomplete. But right now – before phase 3 clinical trials are published and before any raw data has been released, it’s much more imperfect than usual. What normally takes 10 years has been achieved in 10 months, and while vaccine development and testing has been fast-tracked, the biology of human beings remains the same – so you can’t fast track safety.
I’m going to focus here on what we think are the 3 most important things you can do.
1. Sufficient information for properly informed consent
Firstly – you should make sure you have enough information that you think is sufficient for you to exercise your right to informed consent. You have a right to know any information about the safety and effectiveness of vaccines that’s already known to public bodies such as drug regulators that is of overriding public interest. That’s a right that’s expounded in constitutions, human rights legislation and is endorsed by the United Nations Commission on Human Rights. In our book, this should involve at least 2 things: firstly, full disclosure of exactly what’s in each of the vaccines, and that includes the exact composition of the lipid nanoparticles being used to deliver the mRNA in the Pfizer and Moderna vaccines. Are these synthetic or are they animal-based? Do they include shark squalene like a lot of more conventional vaccines – and will animal-derived products like this be injected unknowingly into vegetarians and vegans with vaccine makers and regulators turning a blind eye? The second piece of essential information we think is necessary, is full disclosure of the raw data from Phase 3 trials as well as any other relevant results from safety and efficacy trials to allow independent scientific review.
Presently – we’re a long way from having this – and, quite simply, it isn’t possible to give informed consent without a lot more information being released than what’s currently available. From an informed consent point of view, we’d say right now, on the basis of inadequate information, you have an ample right to ask to delay your decision pending further information. It seems the vaccine lobby is very quick to accuse vaccine hesitants on the basis of claimed ignorance, while it fails to recognise that lack of confidence is largely down to distrust that has built up over years of non-disclosure. The tobacco industry has had its comeuppance for such failure to disclose key information - but the vaccine industry has yet to fall from grace for what is in effect the same failure to disclose information of overriding public interest.
2. Stop governments claiming covid vaccines re safe
You don’t even have to deconstruct the design of the covid vaccine trials or study the available results to-date to have a view on this. You need to just read the trial designs as they stand. The fact that many of Phase 3 trials have primary or secondary endpoints that relate to safety that are months away – requiring 12 or even 24 months of time to have elapsed from the second dose, governments are misrepresenting the science when they claim that vaccines are, or have been found to be, safe. If you combine the use of this false safety claim with ramped up direct-to-consumer advertising, as expected or legitimised in some countries, like the UK, you can get some sense of how prepared governments are to lie to the public. We will all be looking to the courts to resolve any such public health abuses as they occur. But in the meantime – please sign our petition (see below for a selection of petitions you can sign to oppose restrictions linked to refusal of coronavirus vaccines) that asks governments to stop claiming vaccines are safe in the absence of comprehensive safety data. You’ll find all the links to this and other references in the article that accompanies this video.
3. Equal rights for vaccinated and unvaccinated
It goes without saying given the current threat posed by the virus and the uncertainties around the long-term effectiveness and safety of covid vaccines, we are opposed to mandatory vaccination. Mandatory vaccination doesn’t engage with the reasons why so many people lack confidence in the current crop of vaccines. It’s also a major intrusion on individuals’ rights and freedoms, and it undoes all the work in public health that’s trying to develop greater autonomy and responsibility for self-care that’s right at the heart of resolving some of the biggest challenges in health.
What’s actually a bigger threat than mandatory covid vaccination is coercion. This is likely to play out through the withdrawal of rights or privileges from those who don’t consent to vaccination. That might be by stopping those who can’t prove they’re vaccinated from travelling on planes, trains or buses, attending sports fixtures or entertainment, enjoying hospitality, claiming benefits, sending your kids to school – you name it, the list of possibilities currently being discussed in political circles is potentially a long one.
We argue that it’s a infringement on the right to a private and family life to suffer loss of these rights simply because a person has decided there are insufficient data available to give informed consent to vaccination.
Once again, this is something that many of us are watching very closely, and it’s likely that it will be a matter that will ultimately be settled in the courts. But in a world that is rightly calling out for more equality – let me leave you with this last question to ponder: Why are some people so driven to mandate this kind of inequality to those who’ve decided that the lack of vaccine transparency prevents them from making a properly informed choice?
Finally – we ask you to share this video and linked article as widely as you can, on whatever platform works for you. You’ll now find all our videos in the videos section of our website at anhinternational.org forward slash videos. Unfortunately, YouTube isn’t allowing balanced representation of information of vaccines so we can no longer post videos on vaccines on that channel. Bear with us as we navigate the current era of unprecedented censorship and please also sign up to our newsletter for weekly analysis, articles, updates and videos. Thank you.
Other petitions
UK
Prevent any restrictions on those who refuse a Covid-19 vaccination
Germany
Keine covid-19 imfpflicht (No covid-19 vaccination required)
Belgium
Pas de vaccination obligatoire contre le Covid-19! (No compulsory vaccination against Covid-19!)
Canada
Petition to the Government of Canada
If you know of any other petitions that oppose restrictions on citizens who choose not to receive a covid-19 vaccine please let us know.
>>> Find out more about the ANH-Int and BSEM Vaccine Transparency Manifesto
>>> Find out more at ANH-Intl Covid-19 Adapt Don’t Fight campaign page
>>> Please sign up for our FREE ANH-Intl Heartbeat newsletter at the base of our homepage for weekly, information-packed, potentially life-changing information









Comments
your voice counts
04 December 2020 at 7:34 pm
There are two principles which are being ignored or overridden in this rush to vaccinate. They express the same sentiments in different words.
"First, do no harm"; I'm sure you recognize the source of that.
The second is The Precautionary Principle where proof of benignity is required before marketing.
Both are being ignored in this case.
04 December 2020 at 8:58 pm
Thank you for this totally brilliant summary of the key points.
05 December 2020 at 9:15 am
I would like to point your attention to a very important aspects of vaccination to be rolled out in just 4 days.
According to "Information for Healthcare Professionals on Pfizer BioNTech COVID-19 vaccine" https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/940565/Information_for_Healthcare_Professionals_on_Pfizer_BioNTech_COVID-19_vaccine.pdf Pfizer's vaccine is temporary authorised for individuals aged 16 years of age and over. Point 4.6 of above document states "It is unknown whether COVID-19 mRNA Vaccine BNT162b2 has an impact on fertility".
The vaccinations are expected to produce antibodies against spike proteins of SARS-CoV-2. However, spike proteins also contain syncytin-homologous proteins, which are essential for the formation of the placenta in mammals, including humans. Therefore there is a real danger that mRNA vaccines will not only prevent developing of Covid-19 symptomps but will also effectively sterilise vaccinated women.
05 December 2020 at 11:25 am
Why does it say "Private Video - you don't have permission to watch" on the video? Thankfully you've provided a transcript, but I wondered whether Vimeo has also censored the video? Can you upload it to Bitchute or Brandnewtube, perhaps?
05 December 2020 at 11:33 am
Hi Vanessa. It's not been taken down thankfully, just a temporary glitch that we're working to get sorted. The link will work shortly I hope.
I'm so sorry for any inconvenience, but we hope it'll be up again in a couple of hours.
Warm wishes
Melissa
05 December 2020 at 6:55 pm
That's good news. It's working now - I'm beginning to get a bit paranoid about Big Brother these days!
06 December 2020 at 1:53 am
You’ve not actually mentioned any of the risks of SARS-CoV-2 infection/Covid-19. These include death, lung damage, heart damage, kidney damage, liver damage and prolonged Covid-19 symptoms aka ‘long Covid’. Could you add data on these to your article?
10 December 2020 at 9:46 pm
Hi there, you might like to know that we've just published an article on long covid that touches on a number of areas you mention. I hope it gives you some more information from a different perspective. You can read it here: https://www.anhinternational.org/news/long-covid-what-is-it-and-what-might-be-the-best-way-back/
Best wishes
Meleni
06 December 2020 at 8:43 am
Thank you for this excellent summary; I am spreading it as far as I can...
06 December 2020 at 1:10 pm
Thank you Linda. Your support is much appreciated.
06 December 2020 at 10:17 am
Thank you for this essential guide to the key concerns surrounding these vaccines.
I add this link which I also found very revealing.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7142689/?fbclid=IwAR3m3urJXhsBFdH-ymSvwN1MFOEO-YsZVetc1Y5pPcEhzpKpsZRtWwgytgM
07 December 2020 at 10:21 am
Excellent work as usual! Thank you.
10 December 2020 at 5:04 pm
I am new to your site and happened upon it while researching the 2 individuals in the UK with allergic anaphlatoid reactions to the Pfizer vaccine. I have allergies, mostly food (pork and any pork derived product or byproduct), pets, and outdoor. I have only been allergic to pork since 2017, but my last reaction which I am not even sure what caused it ended me up in the hospital with dropping blood pressure etc., which utlimately started me carrying an epipen. I am highly concerned about this vaccination especially with the lack of data. Since now they have advised that those with severe allergies should not receive the vaccination makes my mind wonder even more about it. There are several vaccinations I cannot get already and I am not in any way going to make myself vunerable for another severe reaction.
I know they have released the ingredients, and they say there are no animals products in it, but I am not so sure. I am hoping now that I have found your site, that you guys will be able to determine that possibility. I mean if they say there is no animal or food product in the vaccination, then why is it advised for those who have food allergies to avoid getting it? Thank you for your information, it is very insightful.
16 December 2020 at 2:07 am
Some people can have allergic reactions to a range of different things, and what substances cause them to react can change. The significant factor here is that you do get severe anaphylactic reactions. Maybe the vaccine has some large molecules that could cause problems for people who tend to get serious allergic reactions. Some vaccines are carried in substances that include albumin or gelatin (there's your pork or beef!), or other known allergens. I know someone who had a prolonged reaction (ill for over six weeks, but not an anaphylactic reaction) to one particular vaccine, but not to any others. She is allergic to all sorts of things, including some foods, grass pollen, pet dander, and what she reacts to changes. So she is likely to be refused Covid-19 vaccination. Somewhere online there is a list of what is in the vaccine.
16 December 2020 at 9:00 am
Hi There. Thanks for your comment. We have included a list of the Pfizer vaccines ingredients as we know them above the video in the article above for information.
Warm regards
Melissa
16 December 2020 at 9:18 pm
Hi there, thanks for the video and transcript. Please could you point me towards the original source(s) for the following?
´ in the Phase 1 and 2 trials of the AstraZeneca vaccine, moderate to severe adverse events were experienced by 80% of those receiving the covid jab. With the Pfizer vaccine, nearly 4% of people suffered from severe – or Grade 3 – adverse events.´
Thanks very much
17 December 2020 at 1:50 pm
Thank you for your comment and question.
Dr Verkerk did make an error in referring to an 80% adverse event rate for the Ox/AZ vaccine - it should have been 70%. The figure comes from the Phase 1/2 trial published in the Lancet in July (https://doi.org/10.1016/S0140-6736(20)31604-4), which recorded all adverse events (AE) in the 3 categories: mild, moderate and severe (Grades 1, 2 and 3 respectively). Since the recording was made, the Phase 3 trials has been published (https://doi.org/10.1016/S0140-6736(20)32623-4).
The most common systemic reactions were found to be fatigue and headache lasting several days after first vaccination in particular, both of which can be triggers for downstream neurological or metabolic issues. Fatigue was reported in 70% of a randomly selected subset of participants without paracetemol and 71% of those with paracetamol. These were a lot higher than the equivalent for the meningitis vaccine (MenACWY) 'control' group, which was 41% and 37% with and without paracetamol, respectively. Headaches were reported in 68% and 41% in the with and without paracetamol ChAdOx1-vaccinated groups, respectively. Other common systemic reactions (in the non-paracetamol treated, ChAdOx1-vaccinated group) were: malaise (61%), chills (56%), feeling feverish (51%) and muscle ache (48%).
The Phase 3 trials, including in the accompanying supplementary materials, only reports on 'serious' (Grade 3) adverse events, so it is not possible to comment on mild or moderate adverse reactions given the non-transparency. The serious adverse events were reported in 0.7% of over 11,000 people in the ChAdOx1-vaccinated group, which is on par with the 0.8% found in the meningitis-vaccinated group, itself being far from a safe vaccine. Accordingly, this has meant that the press release accompanying the Phase 3 trial publication in the Lancet does not allude to any adverse events other than the serious ones.
Thank you for bringing the error to our attention. We have now updated the article.
Warm regards
Melissa
05 January 2021 at 4:00 pm
What can I say it's a relief to hear the information you have given.to just try and understand
Some of the issues of the vaccine , as I like most people are simply so confused with what
The government tell us and there scientist tell them . If there is a choice it's a no from me as I have
No faith what so ever in the information that is pushed down our throats and the fear mongering
We have a right to say no this is still our human right we are not China .thank you for giving us
this information .
21 January 2021 at 1:39 pm
Not sure if this will help or hinder your research? The page below has lots of PDF's (you will need to scroll down a little.
https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-december-10-2020-meeting-announcement#event-materials
The UK's MHRA is opaque (to say the least) and we may find other info via the USA's FDA.
Best wishes
21 January 2021 at 1:40 pm
Many thanks for sharing Steve. We'll take a look.
Warm regards
Melissa
Your voice counts
We welcome your comments and are very interested in your point of view, but we ask that you keep them relevant to the article, that they be civil and without commercial links. All comments are moderated prior to being published. We reserve the right to edit or not publish comments that we consider abusive or offensive.
There is extra content here from a third party provider. You will be unable to see this content unless you agree to allow Content Cookies. Cookie Preferences