Content Sections
Anyone daring to suggest that a vaccine might present a risk, especially to children as the most vulnerable members of our society, are usually shot down in flames. Health authorities have tried their best to continue telling everyone that HPV vaccines (note use of plural as there are three different types available) are safe, despite ongoing research suggesting otherwise.
A study just released by a World Health Organization (WHO) monitoring centre in Sweden shows that adverse event reports received from national authorities — and these will represent only a fraction of those actually experienced — show a tendency to produce clusters of serious adverse events that include complex regional pain syndrome (CRPS), postural orthostatic tachycardia syndrome (POTS) and chronic fatigue syndrome (CFS) that exceeds any other vaccine.
GMO vaccine released a decade ago
The genetically engineered vaccine, first introduced for mass vaccination around 10 years ago, has been delivered to around 80 million girls, women and, in some countries, boys.
By August 2014 58 countries had introduced the vaccine into their immunisation schedules. According to the WHO, 80% of all cases of cervical cancer are linked to HPV, the key justification for giving the vaccine to young girls, occur in developing countries and are linked to sexual encounters at very young ages.
Concerns around the safety of the vaccine began to arise in 2013 with reports of CRPS in Japan, POTS in Denmark and CFS in Holland.
This study, which explores global reporting patterns of Adverse Reactions (AEs) for HPV vaccines shows that these different types of effects are often clustered, i.e. they are more likely to occur together, and so are likely related.
The science bit
The study looked at case safety reports for HPV Vaccine in VigiBase, the WHO International database of suspected adverse drug reactions. Reports are received from pharmacovigilance centres in 124 countries who participate in the WHO Programme for International Drug Monitoring up to 1st January 2015. Cases including at least 2 reporting terms were included in the study.
Analysis of data revealed a large number of reports with a pattern of AEs including headache, dizziness, fatigue and syncope (temporary loss of consciousness). Reporting of AEs has taken place since the introduction of the vaccine and increased substantially in 2013 and 2014.
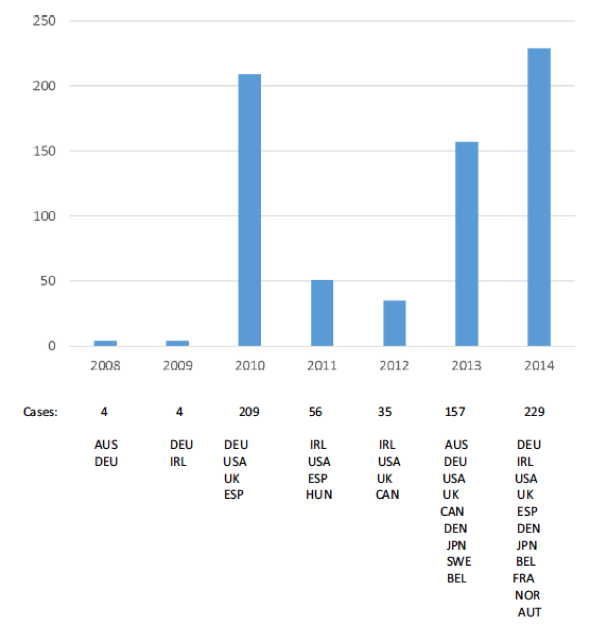
Figure 1. Timeline displaying the number of case reports (per year and by country of origin) that were included in the four clusters. Source: Chandler et al. Drug Saf (2017) 40:81–90.
Researchers used cluster analysis to look at 39,953 HPV safety reports from VigiBase, which were used in the Study. The reports were sorted into 4,116 clusters with 54 clusters containing 5 or more AE reports. The four largest clusters included 71% (28,502) of the safety reports analysed, which reported AE's included on the product label. On average the reports in these clusters only included a few AE terms (3-4), which were generally classified as non-serious.
Review of the smaller clusters included cases related to conditions such as cervical dysplasia (precancerous condition of the cervix), mononucleosis (high fever, swollen lymph glands, and a sore throat) and systemic lupus erythematosus (an autoimmune disease).
Four smaller clusters were found to include terms related to ongoing safety concerns. The most commonly reported AE's were headache, dizziness, fatigue and syncope. In more than 50% of the reports 3 out of the 4 most common AE's were reported. Compared with overall HPV reporting, these clusters included a higher proportion of serious cases (44-89% compared to 24%). Three of these smaller clusters contained a significant proportion of reports relating to POTS (58% - 61/106), CRPS (20% - 15/76) and CFS (37% - 32/87), although these terms were only reported in a small proportion of the total cases included (15% of 694 reports).

Figure 2. Diagram of the four largest clusters and the four smaller clusters of interest. Source: Chandler et al. Drug Saf (2017) 40:81–90.
Further analysis found consistency between the clusters in terms of less commonly reported AEs (such as nausea, muscular weakness, disturbances in attention), diagnostic procedure terms and impact on life including impairment to daily activities, reduction in quality of life and being bedridden. The majority of the cases (77.5% of 694) included in these four clusters were considered relevant to ongoing safety concerns around this vaccine.
The study was limited by the lack of information included in many of the reports in VigiBase, making it difficult to decide on clinical relevancy of reports.
The reported AE's in the clusters were found to be very similar to others described in a number of other safety signals for the HPV vaccines from The Netherlands, Denmark, Japan, US, Mexico and Italy. Commonly reported AEs included fatigue, headache, dizziness, nausea and musculoskeletal discomfort. More serious conditions such as CRPS and POTS and paraesthesia were also found. The number of cases showing similar patterns of AEs across a variety of geographical regions add weight to the overall concerns around the safety of these particular vaccines.
No cause and effect?
As would be expected, the study’s authors comment that the AEs in VigiBase would represent underreporting and there would likely be both undiagnosed and unreported cases of AEs that have not found their way to national authorities and so into VigiBase.
Many studies to date have looked at specific terms in relation to AEs following vaccination with the HPV vaccine, but they did not consider co-reported events in the way this study has.
The cluster analysis used in this study has revealed AEs reported following HPV vaccination that are serious and which overlap in signs and symptoms to recent safety signals for POTS, CRPS and CFS, but that haven't been diagnosed as such.
The pro-vaccine lobby will almost certainly celebrate the author’s statements that the study does not establish any proven causal association between these AEs and HPV vaccination. That is of course no surprise, as it’s merely a cluster analysis of reported AEs and so was not of a design that could establish a cause and effect relationship. Given the nature of the reports, it’s almost inconceivable that most of the nearly 40,000 cases received weren’t related to the vaccines.
This study makes it ever harder for vaccine makers, health authorities, doctors or other health professionals to claim the “safety” of the vaccines – without any qualification. The fact is, serious adverse effects are a real possibility and they may result in weeks, months, years or potentially even permanent effects that can dramatically alter a person’s quality of life.
What should we do now?
We uphold the following:
Health authorities and professionals who vaccinate have a moral if not legal duty to make parents, carers and children fully aware of these known risks, appreciating that known adverse events almost certainly represent only a fraction of those because of lack of adequate diagnosis and under-reporting.
It is also imperative that parents, carers and children are given advice as to what other options are available to minimize the risk of HPV transmission, this centrally being related to avoiding unprotected sexual encounters, especially when under the age of 14.
For more information on HPV and other vaccines, go to our Vaccine Choice campaign page.







Comments
your voice counts
18 January 2017 at 8:10 pm
People should be made aware of any problems with vaccination. The other problem is of course very young children being vaccinated as it does not give their immune system time to develop which will cause a lot more problems in later life.
08 August 2017 at 6:41 pm
is extreme weight gain a side effect of this stupid shot?
24 October 2017 at 1:05 pm
Yes, it can certainly be Darlyn, because the challenge to the immune system can create a level of inflammation in the body, which in turn can impact how the body manages weight. Plus, it depends on how the liver is affected, what vulnerability someone may have genetically, alongside how someone is eating, moving and sleeping. Weight management is a complex issue that is rarely only about food intake.
18 January 2017 at 11:45 pm
On the back of the death of a well known celebrity of cervical cancer the vaccine was pushed onto teenage girls via their schools before parents were made aware of any risks. Disgraceful. Every girl I know of who had the vaccine suffered serious side effects. My niece still has facial palsy.
27 February 2017 at 1:34 pm
Fortunately I figured out some years ago that vaccination is a dangerous fraud.
Unfortunately, most parents are still in a propaganda-induced trance in which they feel compelled to submit their babies and children to be poisoned for profit and to be genetically altered.
04 August 2017 at 7:24 am
Let's not take some.good info gleaned from ONE study, and taint the entire notion of vaccination , as vaccines have CERTAINLY been life saving instruments , even though they definitely need to tske a closer look at these 3.
...
05 August 2017 at 10:53 am
Read Dr Suzanne Humphries' 'Dissolving Illusions' it lifts the curtain on the history of vaccines.
19 October 2017 at 9:05 pm
'CERTAINLY'?? I think you need to study a little more- absolutely NOT certainly.
29 November 2017 at 2:13 pm
Actually, I have seen brain damage in people as a result of measles.... I have also seen disfigurement and paralysis. CERTAINLY they have helped eradicate MANY illnesses that killed thousands. I am still unsure of this vaccine though.....
25 September 2018 at 4:28 am
Measles is hardly eradicated. The measles vaccine has a fairly low efficacy rate as anyone can see from all the vaccinated children catching measles. The measles vaccine also sheds due to it being a live virus so many times it is the vaccinated that spread it. Have you not noticed that when school starts all of a sudden there's a bunch of breakouts of diseases? Because all those kids just got vaccinated and are contagious. It's far from a secret if people just cared to look.
02 August 2017 at 11:16 am
I hope those girls, including your niece, have reported these adverse reactions to the MHRA via their Yellow Card system? The regulators need this kind of feedback before anything will be done about it.
www.mhra.gov.uk/yellowcard
24 August 2017 at 1:15 pm
In my experience it is hard to mention issues when children are very little. The hesitation being the air of authority that a medical practitioner holds, the vulnerability that a parent of a little one generally feels, and the worry that blame will be mislaid at the parent's feet, or more medical unnecessary medical attention will be applied, when a parent is already working on new found responsibility and critical judgment. Don't know what its like where you are?
11 September 2017 at 11:47 am
Robyn Malloy, where do you come from please? So sorry your niece is still suffering serious side effects. Connect with me on facebook at Freda Birrell - I am Chair of our UK Association of HPV Vaccine Injured Daughters (AHVID).
13 September 2017 at 1:21 am
Do you if it causes diabetis1?
20 September 2017 at 8:41 am
Hello Sandra, there are concerns there may be a link between vaccines and the development of auto-immune disease (type 1 diabetes is an auto-immune disease http://www.diabetes.co.uk/type1-diabetes.html). This recent study discusses the issue http://www.sciencedirect.com/science/article/pii/S1043661815001711. This referenced article also discusses the links between vaccination and auto-immunity and may be of interest http://info.cmsri.org/the-driven-researcher-blog/on-vaccines-adjuvants-and-autoimmunity.
I hope this helps.
Warm Regards
Melissa Smith
02 November 2017 at 9:17 pm
My friends daughter developed type 1 diabetes within a short time of having the hpv vaccine and also had all the other symptoms. . Stomach pain, headaches, dizziness no energy etc.. she ended up having a heart attack at 17 years old due to a dvt (most like caused by diabetes) she did not survive... so so sad
04 November 2017 at 5:49 pm
That's is incredibly sad and tragic Helen. I'm so very sorry to hear of this. Thank you for sharing it's important that we collect this information so we can help to challenge the statements being made by health authorities that vaccines are safe.
Warm Regards
Melissa
08 April 2019 at 4:27 am
Was an autopsy preformed and if so what tests were done? She should have been tested for Gardasil adjuvants. The clots, her heart and brain should have been tested for the adjuvants. Was her death attributed to Gardasil?
05 November 2017 at 9:06 pm
One of the adverse affects of the MMR. Is type 1 diabetes
07 October 2017 at 11:30 am
I got the vaccine 10 years ago and I am absolutely fine.
16 October 2017 at 3:33 pm
Unfortunately this can not be said by many young women who have lost their lives as a result of this vaccine. Saying this is incredibly insensitive.
21 October 2017 at 8:53 pm
This is not "incredibly insensitive." This is a single instance of the vaccine not causing known harm. I am reading the information presented because I like to hear all relevant data. Differing or dissenting information does not nullify people's pain. It is her personal relevant input. Stop trying to shut out other people's input or you are attempting to be just as bad as you seem to accuse the government agencies of being.
22 October 2017 at 10:02 am
It is more likely to lose one's life due to cancer that could possibly be prevented by vaccinating.
How is it insensitive to say , as vast majority of those vaccinated, that they are fine, and have a significantly lowered risk to develop dangerous forms of cancer? As a bonus, their protection lessens the risk for their partners.
If there's anything insensitive, it's your scare tactics, Steph.
03 November 2017 at 4:25 am
The science is hardly proven yet that this vaccine has or will ever reduce the chance of getting cancer from HPV. However, studies are evident that the vaccine is causing what it was designed to prevent in the first place. While Sarah may be fine now, we really have no idea how she may be affected later. The risk does not in any way outweigh the benefit that was promised by this poison. Autoimmune diseases are serious diseases that can lead to death, just the same as cancer making your opening statement false!
09 November 2017 at 5:51 am
Am not replying to anyone in particular but want to share my experience with Hpv. Last year at the age of 13 My daughter was given HPV, 3 weeks later we noticed swelling up in fingers. A few months later she was referred to paediatric doctor whose initial diagnosis was athritis and was given a prescription which she reacted to and had breathing problems so she had to stop. To cut a long story short of the events in the past year and half. Her bloods showed high inflammatory markers. Eventually she was referred to the top consultant in Rheumatology and has been diagnosed as Lupus an autoimmune disease. Health officials refuse to link her symptoms to HPV which is very frustrating.
19 October 2017 at 10:06 pm
Thats where I'm confused because there are possible side effects to every medication. Cancer runs in my family even I'm a survivor if I can help prevent cancer hell yes I'm for it!! Thank You Sarah!
26 March 2019 at 4:04 pm
I read that vaccine appears to be more dangerous if given during paramenstrum. That’s just before or just after the period starts. There is an additional risk if other vaccines are received at the same time.
08 April 2019 at 4:37 am
You may not have gotten the same vaccine as those that were harmed. Gardasil varies a lot from one batch to another. You might check the lot number of the vaccine that you received and see how many complaints were against it and compare it with other lots.
26 October 2017 at 6:03 am
My ex forced our older son to get this and he has been suffering from the side effects from the day if the injection. Yesterday she tried forcing our younger son to get it. He refused and in Pennsylvania minors have rights. The doctor tried to talk him into getting it. He said no I stood by him and the doctor at KIDS PLUS IN PLEASANT HILLS PENNSYLVANIA said they probably will not be able to see our children any longer. She also refused to document my concern and verbal request to have our children's medical records list my concerns
26 October 2017 at 11:03 am
Dear David
Thank you so much for sharing your story. How deeply painful for you all and how brave of your younger son to standup for his rights. He's an inspiration for us all. I hope you have found a good functional medicine practitioner to help your older son? If not, you might like to look at the practitioner listing on the Institute for Functional Medicine website: https://www.ifm.org/find-a-practitioner/.
warmly,
Meleni
19 January 2017 at 9:22 am
Good luck in your campaign. I will pass the info to my adult children for theirs.
19 January 2017 at 12:22 pm
Thank you for your support Ray and thank you for passing this valuable information on.
Best wishes,
Susie
19 January 2017 at 4:21 pm
Vigibase Uppsala reported nearly 72.000 adverse reactions of the HPV- vaccine worldwide. They also say that it,s hardly 10 % !!!!!!
www.hpv-vaccin.123website.nl
19 September 2017 at 5:11 pm
Annelies, it is not 72,000 adverse reactions on the Vigibase global database of adverse drug reactions (www.vigiaccess.org), it was 72,000 reports (now 76,399). If you click on the link on the front page for 'adverse reactions' you will find 297,020 adverse reactions, including 400 deaths and 892 neoplasms, inc. cervical cancers.
20 September 2017 at 8:35 am
Thank you for the clarification Steve.
Warm Regards
Melissa
20 January 2017 at 7:56 am
My daughter received her first Gardasil shot at age 14 wiggle she was battling a significant strep infection. She immediately developed severe fatigue, headaches, joint and muscle pain especially in the legs and debilitating anxiety with mood swings. Once a competitive dancer and straight A student she was now bedridden. At age 16 she received her second injection unbeknownst to us. Her previous symptoms worsened and now in addition she had random shocks all over her body. Since these injections she has been unable to work, go to school, drive or dance. She has since been diagnosed with brain inflammation (PANDAS), CFS (chronic fatigue syndrome), CRPS (Chronic Regional Pain Syndrome), Small Fiber Neuropathy, Adrenal Fatigue, POTS and repeat ovarian cysts. These multi symptom issues are now known as Gardasil Syndrome. My daughter received this vaccine because her doctor told her father that if we don't allow her to get this vaccine then we would be responsible for her getting cancer. This started at age 14. She will be 20 years old in February and the best years of her life just keep slipping by.
20 January 2017 at 5:28 pm
Unfortunately the MSM (fake news) and Big Pharma are able to just keep on lying about vaccine safety. My hair dresser said that one of her clients had a daughter about my kids ages, she is 7, and she asked me what I thought was going on with her client's little girl. She said that she was a sweet and normal kid, and since December she has these horrible mood swings that had her crying for 2 hours at a time. Then, she insists her clothes are wet, and can't tollerate the clothes she's worn for months prior. I told my hair dresser that has all the signs of Vaccine Damage (autism symptoms), and I know because by the time my son was 12 months I realized he had autism and I even knew the jab that caused it. Anyways, I told her to ask her if she received a vaccine in the past 6 months, and she texted back that she didn't. Then, moments later she sent my hair dresser another text that she received a FLU VACCINE in October! That was the exact timeline when vaccine reactions like that could be witnessed (2 to 12 weeks).
I looked up a bunch of info on this year"s flu vaccine and sent it to my hairdresser to pass on the info to that client. Unfortunately, she has a little boy, so I'm concerned that he will be autistic since her daughter had a severe neuro reaction and the odds are that her other kids will have issues when vaccinated.
Anyone that is thinking about a Flu Shot, STOP and LOOK UP WHAT THEY ARE DOING WITH FLU VACCINES NOW AND WHAT THEY "APPROVED" WITHOUT ADEQUATE SAFETY TRIALS AND ZERO REAL TRIALS BETWEEN FLU VACCINATED AND NON VACCINATED KIDS (ABSOLUTELY NO SHOT OR PRESERVATIVES GIVEN TO THE NON-VACCINATED GROUP! The CDC and Big Pharma have locked arms and refuse to do any legitimate safety studies on the vaccines given to adults, let alone babies or kids!
They recently added Squalene (an additive that they admit caused the Gulf War Syndrome)! We know the side effects suffered by Gulf War vets include MOOD CHANGES , NEUROPSYCHIATRIC problems
http://articles.mercola.com/sites/articles/archive/2009/08/04/squalene-the-swine-flu-vaccines-dirty-little-secret-exposed.aspx
I pray that the mother takes my information and stops believing her doctor cares about her children. He is just out to get his bonus and refuses to do any research like moms with vaccine injured children have been forced to do! If she takes her daughter back to that idiot then he will convince her that it's not the vaccine, and then she continues to vaccinate her girl and boy and they are damaged far worse than they are now.
I'm an engineer, and I've had more science classes than a family doctor. I've done the research, and every mom's vaccination story that I come accross. These are not coincidences! When these horrible reactions and health problems follow within weeks of a vaccination, then Doctor should be seeing that their is a problem. They are not going to rock the boat and demand to see the studies (that are rigged) and the studies that are independent and performed correctly.
When a 7 yr old girl develops AUTISM AFTER A VACCINE, what more proof do you need that Vaccines cause Autism. Our new doctor even went so far as to admit that ALL autism cases are caused by vaccines. He's not the only doctor, although there are few out there, you can find many that will say the same. In the 1970s they had Autism classified under a vaccine reaction category!
15 November 2017 at 9:08 pm
Keep telling this story! Bring it to schools! Please bring this to schools in Canada, the kids need to hear this truth. I know 4 women who took the ovarian shot and who are now infertile. They cannot have children. I know theres more stories. My dad barely let me get any, however i think i still had the mandatory grade6, grade9 ones maybe. Not sure. It makes me sick thinking about it. But, i get a cold twice a year i think... Thats it. I believe that stress and eating unhealthy have caused my immune system to get sick here n there. But its been rare. I don't need the flu shot. I've barely had the flu. I know im just one person. But im so thankful that my dad at least took initiative about all this when i was a baby. My brother, mom, dad one year probably 1987 i think, took the flu shot. They came all three of them down with hepatitis something and their house was quarantined. From then on dad started reading.
Thankyou dad.
Much love to you all who are suffering from any pains for loved ones.
I am 25, almost a mother maybe one year. I need to be strong
07 August 2017 at 12:01 pm
Please look into Andy Cutler chelation (there's a facebook group). It could be the answer to your daughters problems.
20 September 2017 at 3:55 am
Should she be compensated as this vaccine is imposed / introduced by the regulatory health system
20 September 2017 at 8:34 am
In the UK there is a vaccine damage compensation scheme https://www.gov.uk/vaccine-damage-payment. It consists of a one off payment of £120,000 Vaccine Damage Payment. The applicant needs to show 60% disability linked to the vaccine. It is worth noting that a recent ruling in the European Court of Justice may have paved the way to make it easier for claims for vaccine damage to be made http://anhinternational.org/2017/06/28/european-court-exposes-vaccine-manufacturers-increased-liability/.
In the US compensation claims can made via the National Vaccine Injury Compensation Program - https://www.hrsa.gov/vaccinecompensation/
Warm Regards
Melissa Smith
19 October 2017 at 10:15 pm
Why would you give a sick child a vaccine for something else? Strep alone can get in your bones and possibly kill you!
21 January 2017 at 9:36 am
My daughter had this vaccine in 2012 at 14 she is now 19 and is chronically sick she never made it back to school for an education was to sick and in severe pain threw out her body . She spent a month in a rehabilitation unit her body seized up . They still deny a problem with this vaccine .
The truth will come out
21 January 2017 at 8:49 pm
Have a look at DNRS as a possible path for recovery. This is just a terrible result for your daughter. By the way. DNRS is Dynamic Neural Retraining System.
23 January 2017 at 12:16 pm
I recently watched all of Vaccines Revealed and they interviewed Dr Daniel Pompa who has successfully de-toxed himself (from mercury poisoning) and his adopted son who was autistic. He has a book you can download for free. I don't know if it will work for girls who've had th HPV vaccine but maybe worth trying. So heart breaking hearing all these stories.
02 August 2017 at 11:26 am
Professor Chris Exley of Keele University has been studying aluminium toxicity for about 3 decades. He has been doing research on Alzheimers patients using high-silicon mineral water (eg Volvic and Fiji) to help them eliminate aluminium which is helping their condition
He said that many parents of girls damaged after the Gardasil vaccine have emailed him for advice. He suggested, pointing out that he isn't a doctor, trying this mineral water protocol and said that a few months later, these parents were emailing him again saying their girls were better.
He suggests drinking one litre of that mineral water a day, drinking it all within one hour, and continuing for a week or so. He also drinks it himself every day to ensure that his body load of aluminium is as low as it can be.
Worth a try?
27 February 2017 at 1:51 pm
HPV vaccines are also the most expensive respectively most lucrative vaccines ever. So a cynic's observation could be that one gets what one pays for, even if in the case of HPV vaccines it's just illness, disorders, disabilities or death.
The maiming and killing will only stop when all parents wake up to the fact that vaccines are of no benefit, but only cause.
28 February 2017 at 8:16 am
My daughter has suffered POTS, has Lupus antibodies and has antiphospholipid syndrome after having the gardisil vaccine . It has ruined her teenage years.
05 March 2017 at 11:18 am
Having known your beautifull daughter since a babe in arms, as the bright, vibrant young lady who loves life, it gives me both sadness and anger reading your report. Not anger to you, but the medical profession Can this be reversed? Her father has the ability to make this public
05 May 2017 at 8:20 am
Verschrikkelijk! Deze farmareuzen vernietigen onze kinderen en bij die verschijnselen waaronder neurotische aanvallen, autisme enz willen ze dat mensen hulp gaan zoeken bij een specialist die ook meewerkt daaraan!
Kortom: Een grote massa gifkliniek!
Ook de chemtrails werken daaraan mee om mensen te vergiftigen dus mensen: let op en gebruik je gezonde verstand!
06 May 2017 at 8:03 pm
It has been approved to be given to males now too - do we 'watch this space'?
09 May 2017 at 4:23 pm
The vaccine is actually being given to boys in both Australia and Austria already, there are discussions ongoing in a variety of places around extending the vaccine to boys also, so yes, watch this space!
Warm Regards
Melissa
21 July 2017 at 2:53 am
The US gives it to boys as well
21 July 2017 at 8:48 am
As does Australia and Austria unfortunately.
Warm Regards
Melissa
20 May 2017 at 1:04 pm
In the US the advertising for both girls and boys is prevelant. Both of my children have asked me if they will get cancer if they don't receive the vaccine. I've discussed with them that there are studies that thie vaccine is unsafe and that I was not going to have them get it. What should we tell our children? They need to be informed as much as parents. It is their bodies.
17 July 2017 at 5:59 am
Before I knew better, I had the vaccine administered in my 20's. I did have some serious adverse reactions which forced me to take 3 months of sickl leave from work (and life) and left me debilitated and bedridden. Although my reactions were not permanent (lasting a few months) they were not minor and who knows what residual effects I have been experiencing over the years since my detox. I believe a fully developed adult may be more equipped to fight off the toxins which make up the vaccine which is possibly why my symptoms weren't as severe as some of the young and teenager girls / boys mentioned above. Also, while I cannot be sure, it seems strange that my roommate at the time who also received the vaccine was diagnosed with lupus not too long after the last injection and also developed endometriosis while we were living together.
If your kids are curious, help them to learn how to research for unbiased studies and information regarding this (and all medical related subjects which affect them). It is always better to er on the side of caution and educate oneself to the potential benefits and risks of introducing a toxin / poison / vaccine / pharmaceutical etc into one's body. Make sure to scroll down to the very bottom of any article or scientific study to ensure that the writer or scientists were not paid by the company whose product they are reviewing (this makes their results and opinions biased). Look for independent studies. Look for studies that were conducted over more than 24 months (5~15 years is even better) if possible so that longterm effects can be seen.
Know that the liklihood of contracting cancer without a vaccine is not high. Know that the prevalence of cancer has increased dramatically in the past 30 years in comparison to our parent's and grand parent's generatuons. The more research you do the better you will be equipped to make a thorough decision.
Be aware that any company (pharmaceutical / drug / food / beverage etc) that wants to make a profit is capable of skewing the scientific research results to "prove" their product is effective and harmless. The burden of proof is not on their shoulders (they are protected and subsidised by the very government agencies who are supposed to be monitoring and regulating their activities). The burden of proof is not on the shoulders of the doctor's as they can hide behind the studies which were funded by the pharmaceutical companies and can absolve themselves of any moral integrity by saying that they didn't know it was harmful. If you truly wish to protect your family, take the responsibility to prove the necessity of introducing any foreign substance into your ( or your child's) body upon yourself. Think. Did you get the vaccine when you were young? Do you have cervical cancer? Did your mother get it? Does she have cervical cancer? How about your great grandmothers?
Many of these large companies use fear of the unknown to encourage those who don't know any better (Like myself in my 20's) to purchase their products no matter the cost to your quality of life and even though their product likely causes more harm than good. They have no social conscience or ethical / moral imperatives. They have lots of lawyers who can spin their lies into plausible sounding truths and suppress bad publicity. They have shareholders who demand increased profits at every quarterly review so their goal is to profit from the willfully naive.
Trust your gut, do as much unbiased research as possible and protect your family.
Best wishes to you all.
17 July 2017 at 1:18 pm
Thanks for your comments Anika. We wish you well for the future.
Warm Regards
Melissa Smith
27 October 2017 at 7:50 am
Hail You Anika ~ I hope millions around the globe read your advice!
21 June 2018 at 4:03 pm
Thank you Annika for your info.
21 June 2018 at 4:04 pm
Thank you Anika for your information.
02 August 2017 at 11:31 am
The Cancer Research page puts it quite clearly that most people will be infected with some kind of HPV during their lifetime, and the body is capable of eliminating it within a couple of years at the most, as long as the immune system is strong. Stopping smoking (or not starting) is a hugely beneficial way to improve immunity, as is eating a diet with the optimal levels of micronutrients (especially magnesium, zinc, vitamin C and vitamin D - ideally along with sun exposure at the right times of day/year), finding ways to deal with stress (eg meditation, mindfulness and adequate magnesium intake), avoiding sugar and refined carbohydrates which compromise the white blood cells etc.
All of these things are what should be taught to our children, not that their bodies and immune systems are faulty and need the 'help' of things like vaccines!!
02 August 2017 at 1:40 pm
This is indeed, a key point Vanessa. That HPV infection very often resolves itself without need for further intervention.
Warm Regards
Melissa
29 May 2017 at 1:04 am
My 15 year old, extremely active, competitive dancer daughter fell ill in August 2015, nausea, dizziness, vomiting, headaches weeks after her 3rd Guardasil shot. After the 1st two shots she complained of headaches but never put 2 and 2 together. Several ER visits several hospital stays, more issues, fatigue, trouble waking, ptosis, high blood pressure, high heart rate, losing weight despite decent appetite, hiccups, severe itching where nothing alleviates, choking, sleep problems. Trouble feeding herself, she was incorrectly diagnosed several times at the hospital. CT scans, MRIs, PET scans, diagnosed with pheochromocytoma,, surgery done in Jan 2016 to remove left Andrenal. No pheo but pathogy report said it was adrenal medullary hyperplasia. Had full genetic testing done as well, no genetic component to this. I have asked several drs how she got this, no one can give me an answer. I am convinced it was due to her HPV vaccine. She has recovered after about 7 month from onset but now I am being asked by school to get her meningitis vaccine.
31 May 2017 at 4:53 pm
Thank you for sharing your story Kristin. We're very sorry to hear about your daughter's experience it must've been a difficult time for you all.
Warm Regards
Melissa
18 June 2017 at 10:32 pm
I wish I never trusted my daughter's pediatrician, who told me this vaccine was going to be like the next Polio vaccine. Ever since she received these vaccines, I noticed a difference in her behavior. My sweet, loving girl became someone I barely knew, and isn't it a coincidence that this started with this vaccine? I sincerely wish I could turn back the clock, because I am convinced the vaccine altered her personality.
19 June 2017 at 8:12 am
Hello Deb
We're very sorry to hear how the HPV vaccine has affected your daughter. We've heard many stories such as yours and it's very concerning.
We wish you and your daughter well.
Warm Regards
Melissa Smith
13 July 2017 at 5:26 pm
I have twins that received the Gardasil vaccine at the same time. Twin A developed mononucleosis. Both twin A and B developed mild dysplasia and twin B has had headache sometimes migraine 24/7 since shot. I thought I was doing something good for my children. Now we just try to focus on getting better.
14 July 2017 at 7:11 am
Hello Em
We're really sorry to hear you've had such a negative experience with the Gardasil vaccine. We hear so many stories about health problems following this vaccine, but the health authorities continue to dismiss them. We will continue to campaign on this issue.
We wish you and the twins well.
Warm Regards
Melissa Smith
01 November 2017 at 9:46 am
My 12 yr old daughter got the shot a month ago and started having body aches immediately. She is
Currently hospitalized with all kinds of issues. Strep, possible mono, 105 temps, high blood pressure,facial and lymph node swelling,belly and spinal pain and nausea. I am convinced the injection is at fault. She has never been sick. Doctors can not figure out what it is.
13 July 2017 at 11:58 pm
Once again I had to defend my choice to opt out of this vaccine to my daughter's new doctor. She even went so far as to say "if i could take a vaccine to keep e from getting cancer I would." It took everything in me not to tell her then by all means vaccinate yourself! At least my old doctor respected my choice but this crow tried to make me feel like a negligent parent. Thankfully it didn't work because I know I'm a good parent that has MY child's best interest at heart. Until children stop suffering after taking this vaccine I will never consent & I'm educating my children in how to make informed decisions for their health. Now I must find another doctor. One that respects a parent's decision AND isnt bought by big pharm. There was entirely too much paraphernalia about this vaccine all over the place so I was prepared for this b.s.
14 July 2017 at 7:13 am
Well done on standing firm and defending your daughter's health. We hope you can find a more understanding Dr to support you in future.
Warm Regards
Melissa Smith
17 July 2017 at 6:10 am
Well done. I admire you for following your gut and doing what is right for your family. I know I will refuse to vaccinate when I have my own children. They have done nothing more than cause constant illnesses in my life and I wouldn't wish that suffering upon anyone.
It certainly is important to align with a medical practitioner who isn't being bought or bribed by the pharmaceutical industry. Good for you!
Best wishes!
17 July 2017 at 1:18 pm
Thank you for your support Anika.
Warm Regards
Melissa Smith
20 July 2017 at 1:50 am
In 2014 I got the vacine and for a couple of weeks nothing was going wrong but after the two weeks I started getting dizzy and breathes if I sat up, stood up or walked a few months of this and it continued to get worse I went to the doctor who told me my heart was too fast after several tests and poking and prodding I was told I have pots syndrome and started on medication. The sad thing is that before that injection the only time I was in hospital was for a broken finger. I was never sick before and never got a cold, now I'm played with dizzy spells and I get sick more. I am all for a vacine that helps but this is hurendos I mean two people in the room I was fainted from it they had mats on the floor for them and another 2 or 3 were vomiting.
20 July 2017 at 7:47 am
Thanks for sharing your experience Nikki. We're sorry you've so badly affected. It's stories like yours that keep us doing what we're doing to make people aware that vaccines are not as safe as they're made out to be.
We wish you well.
Warm Regards
Melissa
20 July 2017 at 6:17 am
Im Worried i have made a mistake with my daughter just receiving the first shot last week. We did read up on the information. But focused on the fact sites and not people. She is doing ok, but I am very worried i may have just voluntarily harmed my sweet girl. We will not get the second shot, and I pray she will still be the normal baby I have always had. I wish I read parents posts instead of just ignoring them.
20 July 2017 at 7:50 am
Hello Amber, part of the problem is that a lot of the adverse reports are not being accepted by the health authorities so problems with the vaccine are being masked and not shown not included in 'official' information.
Thank you for sharing your thoughts. We hope your daughter continues to be well. Please share our message so other parents can make an informed decision about whether or not to consent to this vaccine for their children and consider signing our petition if you haven't already.
Warm Regards
Melissa
23 July 2017 at 6:22 am
My daughter had the vaccine at age 12 and developed warts. No one in our family has ever had warts and now, her Dermatologist can't get rid of them faster then ever more sprouting in another area. Someone needs to do a class action against these people.
24 July 2017 at 8:41 am
Hello Loraine. We're very sorry to hear your daughter is experiencing such problems following the HPV vaccine. Thank you for sharing your story with us.
You may also find the UK Association of HPV Vaccine Damaged Daughters a good resource and place to get support from other parents whose children have suffered adverse reactions as a result of this vaccine https://www.facebook.com/AHVID.UK/.
Warm Regards
Melissa
07 August 2017 at 12:48 am
Try a really good oregano oil or Apple cider vinegar on some cotton wool then leave it on overnight with strong tape. It worked a treat for me
09 September 2017 at 3:44 pm
To Lorraine,
Are you sure it isn't mullucus contagious.
26 July 2017 at 1:41 pm
Is there studies showin that people had onset symptoms almost 5 years after the 3rd shot?
01 August 2017 at 6:08 pm
The vigiaccess data-base appears to make it difficult to evaluate data.
For HPV vaccine there are now (August 2017) 76,154 reports and the summary page of Adverse Drug Reactions (ADR's) shows 167,919 including 300 deaths listed under 'General disorders'. I have analysed details of each category and found over 282,000 adverse reactions recorded and at least 378 deaths. Adverse reactions are also reported under a huge number of categories (almost 6,000) without any attempt to group them (eg. at least 30 different types of rash). Reactions at the injection site are reported separetley for injection site, vaccination site, application site, etc, etc.
I have groupe dome of the reported reactions and found at least 34,092 reports of 'systemic pain', 24,623 reports of Autonomic problems, 9,784 reports of rashes, 7,261 reports of fatigue/sleep disorders, etc, etc.
I have written to the Uppsala Monitoring Centre and they have confirmed that deaths are not all recorded under 'general disorders'. They have also acknowledged that only approximately 10% of adverse events are reported.
02 August 2017 at 8:10 am
Thank you Steve. We'll take a look at your analysis and look forward to chatting with you further.
Warm Regards
Melissa
02 August 2017 at 6:29 am
My daughter had the series of HPV vaccines and has been in constant pain for over eight years. It started in her stomach and has progressed throughout her body. She also developed POTS and weakness in her legs and constant headaches and migraines. She was not able to finish school and is unable to work. She has to spend a lot of time in bed because of her health problems. Sometimes she will go out with friends but it takes days to get back to her "normal" am on t of pain. This vaccine is poisoning so many young people and it is criminal that it is still on the market.
We are lucky that we found a FB page with over 1,300 other parents whose children have been injured and we have gotten a lot of information to try to help my daughter get better.
02 August 2017 at 8:13 am
Thank you for taking the time to share you experience of the HPV vaccine. We're very sorry to hear of the problems your daughter is experiencing. This is indeed a debilitating vaccine and we will continue to fight for parents and children to receive proper information on so they can make an informed decision to vaccinate or not.
You may also find the UK Association of HPV Vaccine Damaged Daughters a good resource and place to get support from other parents whose children have suffered adverse reactions as a result of this vaccine https://www.facebook.com/AHVID.UK/.
Warm Regards
Melissa Smith
02 August 2017 at 11:37 am
Having not long ago worked in a hospital department where many teenage girls were being treated for chronic fatigue syndrome and similar conditions, I could never understand why the doctors treating them never asked the question, "What happened just before you got the problem?", or specifically asking about vaccinations, given that there is so much media attention to this HPV vaccine and problems reported in the press.
Doctors either don't want to make the connection or it never crosses their minds that there is any problem with any vaccination. The only thing reported in all children's case notes (for any condition, autism, learning disorders, asthma, eczema etc etc) is "'so-and-so' is fully up-to-date with their vaccinations". That's all they seem to care about!
09 August 2017 at 2:24 am
Personally I NEVER gave my children that vaccination, I hate myself for vaccinating them period, it's just so sad when parents, especially your child's pediatrician, judges you for the choice you made.... It's my descision, not society's!!
15 August 2017 at 10:36 pm
My 14 year old daughter has just gone through the worst year of her life after suffering the side affects of this injection. I would be so grateful for any FB pages or information of ways to help my daughter get better.
16 August 2017 at 8:04 am
Hello Debbie, thanks for your comment. We're sorry to hear your daughter has suffered so badly following being vaccinated against HPV.
The UK Association of HPV Vaccine Damaged Daughters is a good resource and place to get support from other parents whose children have suffered adverse reactions as a result of this vaccine https://www.facebook.com/AHVID.UK/.
We send our best wishes to you both and hope things improve for you both.
Warm Regards
Melissa
16 August 2017 at 9:23 pm
Any studies/reports on children born to a mother that had the shots
17 August 2017 at 11:15 am
Hi Lorrie - we are not aware of any studies as of yet.
- Kind regards Miranda
16 September 2017 at 8:30 am
I had the hpv gardisil when I was in my early 20s. I'm now 31, I have been trying to fall pregnant for nearly 2 years and my naturopath said females who have had the gardisil shots have a hard time getting pregnant. And I still had a case of a low grade hpva few years ago which went away on its own but I don't think the shots did anything
16 September 2017 at 8:31 am
Except make me infertile :(
19 September 2017 at 9:23 am
Hello Lisa, thanks for your comments. We're really sorry to hear about the problems you're having. There are a lot of concerns around adverse effects from the HPV vaccine, but very little research has been done as yet so we can't say if difficulty conceiving is directly linked.
We wish you well for the future and hope your naturopath can help you overcome these problems.
Warm Regards
Melissa
17 August 2017 at 4:19 am
Is there any studies that have any info for someone who had all 3 of the shots when they first started giving the shots and now they have children of their own now and it could of affected the children???
17 August 2017 at 11:16 am
Hi Jessica - we are not aware of any studies as of yet.
- Kind regards Miranda
27 August 2017 at 7:15 pm
Hello, I'm From Mauritius. My daughter of 11 is suffering from chronic head pain from morning till night after the cervarix hpv vaccine. It's almost 1 year. We have so many gymnastic with the doctors at the hospital, doing all sort of test (X-ray, blood test, ENT, eye test, MRI, EEG, cupping, visited psychologist & neurologist & neurosurgeon, nephropathy, acu-pressure) and they are not admitting at the hospital that this chronic headache is associated to the HPV vacine. We are not knowing what to do. She even cant go to school and is unable to learn.
29 August 2017 at 9:12 am
Hello Abdullah. We're sorry to hear of your daughter's health problems. It must be very stressful for everyone.
Although based in the UK, you may find the Association of HPV Vaccine Damaged Daughters good resource and place to get support from other parents whose children have suffered adverse reactions as a result of this vaccine https://www.facebook.com/AHVID.UK/.
We wish you well for the future.
Warm Regards
Melissa
01 September 2017 at 7:56 am
If your daughters mood starts changing around the age of puberty then do not be surprised, it's called adolescents.They can be funny little buggers at this time, lazy , always ill, moaning, you know it's in all the history books. This report has not stated a chance pattern, ie 250,000 to one chance there could be a problem, but think of this. hpv is a virus, when the kids are given it boys or girls they are give a bit of the virus itself to help kick in the immune system and neutralise the virus so that the 80 million people do not get cervical cancer. we have a 1 in a hundred chance of getting run down crossing the road, so shall we stop crossing the road, shall we stop getting into planes, shall we etc etc. I am telling my son to not have sex with some one who is harbouring hpv just like since the false reports about mmr vaccine, don't let the kids who don't have the vacine play with the kids who have not had the vacine yet.
05 September 2017 at 5:47 pm
The WHO do not say the above statements. Here is a link to their most current findings.
http://www.who.int/vaccine_safety/committee/topics/hpv/June_2017/en/
Please don't let yourselves be convinced by science that isn't based on real facts but rather coincidence. These syndromes do not show greater in girls who have had the vaccine than those who haven't. When they do show it happens to be around the same age as when the vaccine is administered.
This is so reminiscent of the MMR vaccine scares that all came down to lies and coincidence but caused deaths from diseases that otherwise would have irradiated.
20 September 2017 at 6:44 pm
Philip, I do not rely on coincidence, hearsay, gossip, anti-vaxx sites or Dr Google for evidence and true facts. However, I also don’t rely on public ‘head-lines’, ‘summaries’ and ‘propaganda’ from health authorities or the vaccine manufacturers. Instead I read the detail from the vaccine manufacturers and ask questions in parliament or make freedom of information act requests to the health authorities, even then you have to pick your way through the ‘smoke and mirrors’.
We are usually told that vaccines are ‘safe and effective’ and have undergone rigorous trials but I can provide you with a list of at least 14 or more that have been withdrawn from the market because they caused meningitis, Lyme disease, seizures, etc, or were contaminated. I haven’t included GSK’s Pandemrix flu vaccine, no longer recommended for children, since it can cause narcolepsy. More realistically, but still not necessarily truthful, we are told ‘the benefits outweigh the risks’ but even this can’t be said for HPV vaccines because there is zero evidence that they will ever prevent a single case of cancer, even the manufacturers only state that they are ‘intended to’ or ‘expected to’. Actually, Cervarix is ‘intended to’ prevent just 2 strains of HPV and Gardasil just 4 strains but there are at least 170 strains known to exist and 40 known to be involved in cancers. Gardasil 9 isn’t used by the government yet but it still has a long way to go. There are scientists who are sure that other strains, possibly more lethal, will fill the void by those strains possibly prevented by the vaccines.
The MHRA publicly states ‘no serious risks have been identified associated with HPV vaccines in the UK’. However, when asked a question in parliament they admit that there have been 3,038 serious reports raised by Yellow Card, see http://www.parliament.uk/business/publications/written-questions-answers-statements/written-question/Commons/2017-04-13/70973
We are also usually told that the side-effects are mild and short-lived but their answer in parliament recognised 40% of Gardasil reports are serious and 60% of Gardasil 9 reports are serious. They even acknowledge that only 1-2% of adverse events (AE) and c.10% of serious adverse events (SAE) are reported. Similar requests by FOIA to the MHRA reveal over 22,000 adverse reactions reported, including 8 with fatal outcome. The WHO also has similar information on its little-known global database of adverse drug reactions at www.vigiaccess,org which lists 400 deaths and 892 neoplasms (inc. cervical cancers and pre-cancers) amongst the 297,020 adverse reactions in 76,399 reports. Presumably you will believe all of these are coincidence despite the vaccine manufacturers listing many of these side-effects as ‘known side-effects identified during the clinical trials’ on their patient information leaflets (PIL).
With respect to the WHO and GACVS claim that HPV vaccines are extremely safe, you only have to look at who the authors are that have provided the so called ‘evidence’. They are virtually all funded directly or indirectly by pharmaceutical companies or other stakeholders who benefit financially from the sale of this vaccine. I would also refer you to the open letter from research pathologist Dr Sin Hang Lee to the Director-General of the World Health Organization, Dr. Margaret Chan regarding scientific misconduct by the GACVS https://www.scribd.com/document/298575313/Allegations-of-Scientific-Misconduct-by-GACVS It also provides significant factual evidence. Similarly a complaint by Cochrane Nordic has been sent to the European Medicines Agency (EMA) over maladministration at the EMA, see: https://nordic.cochrane.org/sites/nordic.cochrane.org/files/public/uploads/ResearchHighlights/Complaint-to-EMA-over-EMA.pdf
With respect to your claim that ‘these syndromes do not show greater in girls that have had the vaccine than girls who haven’t’, I presume you refer to the MHRA’s and WHO’s evidence which supports this claim, a study by MHRA authors inc. Katherine Donegan and Philip Bryan ‘Bivalent human papillomavirus vaccine and the risk of fatigue syndromes in girls in the UK’, https://www.ncbi.nlm.nih.gov/pubmed/24001935 If you read the detail of this study it was based on statistics with seriously flawed and wrongful assumptions, even the authors raise their own concerns. It compared reports of patients with CFS/ME-like diagnosis raised within 12 months after vaccination with the diagnosis of patients, in the same population, more than 12 months after vaccination. It failed to grasp that the average time for a diagnosis of CFS/ME in the UK is over 3 years. It is accepted that CFS/ME-like illnesses are not only caused by vaccines. They are known to be typically caused by exhaustion from overwork, repetitive infectious disease, recent immunisation, significant illness or trauma and toxic chemical exposure. With the HPV vaccine it is a double whammy. As well as immunisation it also includes aluminium, a known neurotoxin. I prefer to rely on the evidence of Rebecca Chandler, even then I believe this only represents the tip of the iceberg: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5209415/
P.S. with respect to the MMR vaccine scare being a lie and coincidence, don’t be fooled. The original MMR vaccine contained mumps Urabe strain which also caused meningoencephalitis. It was eventually replaced by MMR vaccine containing mumps Jeryl Lynn strain
05 September 2017 at 11:27 pm
I've got one of those shots and now my mom knows how bad they are so I'm not getting anymore. Luckily I didn't get any symptoms from the first one :/
06 September 2017 at 8:58 am
Hello Briana, it's good to hear that you weren't affected by the shot you had and that your Mum found out more information so she could make an informed decision about future shots.
Warm Regards
Melissa
06 September 2017 at 9:41 pm
How interesting that none of you responded to Philip's very sane and up to date reference from the WHO. Presumably you only believe them when they agree with you. Vaccines do not cause autism , they save lives. Your stories of ill children are very sad and tragic but there are also many many parents grieving over children lost or damaged by measles,influenza, and cervical cancer. We are beyond lucky in the UK to have vaccines offered to our children on the NHS.
Flo
23 September 2017 at 5:01 pm
Flo, not everyone agrees with you in the debate in BMJ and most of them are health professionals: http://www.bmj.com/content/358/bmj.j4100/rapid-responses
20 September 2017 at 8:25 pm
Flo, I have now responded to Philip’s comment but forgot to mention another really serious concern with the HPV vaccine. There is zero evidence that it will ever prevent a single case of cancer but there certainly appears to be evidence that it can actually cause cancer. As well as the huge number of neoplasms, including cervical cancers and pre-cancers, reported on the WHO’s database of (suspect) adverse drug reactions at www.vigiaccess.org , the UK government statistics websites at ONS (England) and ISD (Scotland) are reporting increases in cervical cancers in the 18-24 years age groups in recent years. This is despite the fact that the majority of these young women should be vaccinated (the vaccine was introduced for 12/13 year old girls in 2008 with a 3 year catch-up programme for girls up to 18 which was so successful it was completed in 2 years). Although the increase in England is only slight surely it should be reducing. However, in Scotland the increase in cervical cancers (C53) is quite significant. From 1994 to 2005 there was an average of 6 cases per year. From 2006 to 2008 (just before the vaccine was introduced) the number of cases averaged just 3/year. However, from 2012 to 2015 (when many of the 20-24 age group should be vaccinated) there has been an average of 11 cases a year, nearly 4 times more than before the vaccine was introduced!
Perhaps this should not be a surprise since the HPV vaccines are the first of a new breed of genetically engineered (GM) vaccines which:
• have not been assessed for carcinogenicity.
• Virus strains prevented by the vaccine might be replaced by more lethal strains.
• Presence of aluminium bound recombinant DNA (rDNA) in Gardasil, the consequences of which are unknown.
• In addition to cervical cancer there are indications that HPV vaccines may also increase risk of other cancers, for example leukemia. The observed number of cases of acute leukemia after Gardasil vaccination exceeds the expected number according to an EMA assessment report: EMA/CHMP/76591/2015, Procedure No. EMEA/H/C/003852/0000.
What is more, at least the Scottish government appears to have taken its eye off Pap screening while it focuses on vaccination. From 1990 to 2007 the all-age (total) cervical cancer annual rate reduced consistently and progressively from 500 cases per year to less than 300 cases per year as screening rates increased to approx. 80%. Since the vaccine was introduced in 2008 screening rates have reduced and cases of cervical cancer have increased consistently and progressively back up to 400 cases per year. An increase of 100 cervical cancers per year!
Considering that HPV vaccines are frequently inappropriately termed “cancer vaccines”, emerging signals that the vaccines may actually cause a cancer epidemic may become a tragic stroke of fate.
21 September 2017 at 10:43 pm
My daughter got the vaccine 8/2016 and two weeks after the second shot she started to complain about fatigue, headaches, sore throats, fevers and joint pain. Her doctor kept testing her for strep and mono. Her doctor still doesn't believe it was the vaccine. I wholeheartedly believe it and the more I research the more upset I get. This information should be yelled from the rooftops. It's been 1yr. My daughter is still having problems she's been diagnosed with reactive arthritis which is an autoimmune disease. She also developed nodules on her throat. We started going to a functional medicine at the Cleveland clinic. She takes supplements eats organic, no dairy or gluten which seems to help. I reported this to the CDC but I couldn't get the doctor to. I'm going to try the Fuji water I have not heard that before. She is right now doing a chelation which I pray will work.
05 November 2017 at 1:28 am
My daughter is 12 two weeks after the vaccination she got puss on her throat, fever, joint aches, and went to the hospital twice, they couldn't figure out what was wrong and just kept treating her for upper respiratory infection and a "virus" and finally they gave her a steroid which seemed to give her some relief, she is still complaining of joint pain, her stomach hurting, her face turning bright red and just feeling tired and not good! Makes me so sick I gave her this shot! I will not be getting any other rounds of it ever!!!!
23 September 2017 at 11:44 pm
INEFFICACY OF THE HPV VACCINE SEEN BY DOCTOR OF DEEP PERÚ
From its inception until the appearance of uterine cervical carcinoma (UCC) takes a average of 25-30 years; the research of this vaccine have begun in 2000. It is evident that the scientific efficacy of this new vaccine will be determined the years 2025 – 2030.
HPV not causes definitely the (CCU); at the onset of this disease involves multiple risk factors, including the suspected HPV, but scientifically is proven by epidemiology and statistics that the sex is what generates this disease. Nix in 100.000 nuns found not any UCC.
http://www.portalesmedicos.com/publicaciones/articles/1832/1/Epidemiolog.,.
There are not scientific researchs; stadistic, epidemiologic, citologic, histologic, colpocopic and clinic to demostrate that the HPV produce the cervical cance, are publishing. whitout scierntific sustentance.
To accept that a virus or a bacteria causes a infection disease must unfailingly fulfill the five Koch's postulate
http://www.xatakaciencia.com/salud/los-postulados-de-koch
1 - The agent must be present in every case of the disease and absent from healthy.
2 - The agent must not appear in other diseases.
3 - The agent to be isolated in pure culture from disease lesions.
4 - The agent of causing disease in a susceptible animal being inoculated.
5 - The agent must again be isolated lesions in experimental animals.
http://es.scribd.com/doc/44558220/MICROBIOLOGIA-1
Consequently, HPV not fulfill not any principle of Koch's postulates. by not meeting this postulate, that is accepted as dogma in medicine, scientifically we must be ensure that the HPV is not the causative agent to the UCC..
Until May 2013 Vaccine Adverse Event Reporting Syntem (VAERS) published that the vaccines against the HPV caused only in Unites States 138 muertes and 30020 adverse events; 947 disabled: 12 males, 924 females and 11sex unknown; 4050 advers graves: 106 males, 3883 females and 57 unknown sex; 527 abnormal PAP smears, 214 dysplasia cervical and cervical cancer 214. Vaccine Adverse Event Reporting System secure that only the !% to 10% are denounced https://dub104.mail.live.com/default.aspx#n=1521802 http://holyhormones.com/vaccinations/hpv-vaccine/hpv-vaccine-adverse-eve...
http://therefusers.com/?s=cervarix
The Vaccine efects advers reactions (VAERS) ensures that only complaint between 1% to 10% of the adverse effects produced by this evil vaccine;this figures shown are calculated according to the statements of the VAERS: to 10%.
http://www.noticiero.enkoria.com/2011/diez-menores-que-sufrieron-reaccio...
http://www.pop.org/content/merck-researcher-admits-gardasil-guards-again...
Dr. Harper, who contributed to the development of the vaccine by Merck, reports that the vaccine was not investigated in children under 15 years and the vaccine given to children under 11 years is a big public experiment.
http://offtheradar.co.nz/vaccines/53-researcher-diane-harper-blasts-gard...
The vaccine was approved to give girls uncontaminated with HPV, Dr. Howenstinc ensures that the women are vaccinated with HPV contaminated, have the possibility to acquire a 44.6% CCU
http://www.newswithviews.com / Howenstine/james170.htm.
Merck did not disclose that the vaccine was transgenic, the Sane Vax has discovered, which is transgenic because it has been found that the vaccine is contaminated with DNA recombinant vaccine Gardasil (DNArPVH) and has raised its concerns to the president of the FDA Margaret Hamburg. The FDA replied that the vaccine will not cause any damage transgenic
http://real-agenda.com/2011/09/16/vacuna-gardasil-contaminada-con-adn-re...
http://bolsonweb.com.ar/diariobolson/detalle.php?id_noticia=26075
A vaccinated child was ill with rheumatoid arthritis, which is an autoimmune disease. 24 hours after vaccination and found that the aluminum adhered to DNArPVH, two years after vaccination and in autopsy 6 months after death in a New Zeland girl Jazmine Renata which had recibed this deadly vaccines
http://www.mecfsforums.com/index.php?topic=9331.0
Management time to get market approval of a drug the FDA is at least three years, it is a drug for cancer 15 years, but the authorization Merck had only six months and the European Medicines Agency (EMA in English) only 9 months: To introduce the vaccine are using the marketing of fear
http://mujeresenaccion.over-blog.es/article-vph-la-vacuna-del-marketing-... http://mujeresenaccion.over-blog.es/article-vph-la-vacuna -of-marketing-of-fear-67210961.ht
HPV is ubiquitous; lives in wild and domestic animals, pollute us from birth, is on the doorknobs, on towels, on nails, on fomites, in gloves and specula of gynecologists,. sexual intercourse is not the only means of contamination.
http://spa.myhealthygood.com/cancer-cervical-vacuna-contra-el-vph/invest...
HPV also lives in the 400 nm outermost of our skin and mucous membranes. ,
If it live in our skin, our immune system produces cellular and humoral immunity is acquired or that our body is self vaccinatinge by PVHs living on our skin and mucous ..
http://www.conganat.org/seap/bibliografia/HPVToday/HPVToday007SEAP.pdf
The PVHs is not distributed uniformly worldwide. It has been found that in Canada HPV 18 only reaches 3%; is more often HPV 31, in my country Peru no studies have determined that HPV types predominate; Gardasil contains 225 mcg. aluminum and Cervarix 500 mcg, that produce the Alzheimer, Parkinson and autism, produce too neurotoxic and immune system disorders (Blaylock 2012) and polisorbato 80, a powerful contraceptive, that in experimental animals produces sterility, atrophy of the testicles and disturbance organic and funtional of the organs of the reproduction; is carcinogenic and mutagenic; also contains sodium borate considered poison unused in medicinal preparations (NLM)
http://www.telefonica.net/web2/paramahamsa/vacunaninosalerta.html http://detenganlavacuna.wordpress.com/2010/11/09/gardasil-cervarix/
Have been discovered to date 200 types of HPV; HPV is not infectious, contagious; the intercourse is not only that the persons is contaminated
http://quimicaclinicauv.blogspot.com/2006/08/virus-del-papiloma-humano.html http://www-lab.biomedicas.unam.mx/smpv/queeshpv.htm
On 22-11-2010 FDA approved Gardasil for males aged 9 to 26 to prevent warts and cancer to the anus, is overkill
http://real-agenda.com/2011/09/16/vacuna-gardasil-contaminada-con-adn-re...
http://salud.aollatino.com/2011/02/02/aprueba-fda-nueva-indicacion-vacun...
For the reasons from deep Peru Huancayo, I believe that this vaccine is a fraud?, a robbery?, a swindle?, a rough joke?, a crime?, a shame?, a scam?
The HPV is not scientifically proved for the moment that produce the UCC its effectiveness shall be verified just the years of 2025-2030.
Dr. Godofredo Arauzo
E mail: [email protected]
25 September 2017 at 9:18 am
Thank you for such a detailed comment Dr Arauzo. Your support is much appreciated. It's only by working together that we can be heard and get the safety of this vaccine reviewed.
Warm Regards
Melissa Smith
09 October 2017 at 2:22 am
Melissa,
Please can I have a contact phone number for you, or can you phone me on 0419 230 456.
My daughter has contracted MS thru this Gardasil drug!
Regards
Sherry
10 October 2017 at 1:48 pm
Hi Sherry, If you would like to call the office on 01306 646600, I would be happy to put you through to Melissa, but not on a Friday as she is not in the office on that day.
With kind regards
Miranda
12 October 2017 at 1:59 pm
hi guys
Really worried about this HPV vaccine as it will be offered to my daughter in 2 years in Ireland and would like to read real facts.
I have tried this site www.vigiaccess.org as mentioned earlier in a post but failed to access the data regarding side effects etc
Can someone advise please as when I enter HPV in the search it turns up nothing
16 October 2017 at 9:57 am
Hi Sharon
There is a Facebook page run by Irish parents, which you may find of help - https://www.facebook.com/BlockedByTheIrishCancerSociety/ plus there is a organisation called Regret set up parents of HPV damaged children in Ireland - https://www.facebook.com/REGRET.ie/. You can also contact the Association of HPV Vaccine Injured Daughters, based in the UK and run by Freda Birrell - https://www.facebook.com/AHVID.UK/.
I hope these help. If you need anything else please email me on [email protected]
Warm Regards
Melissa
21 June 2018 at 4:38 pm
Hello Melissa.
Thank you for all the information and Links. My name is Claudia, another mother in Ireland with a daughter that has had 3 Gardasil vaccinations between sept 2013 and may 2014. Her symptoms did not start straight away so i did not connect it to HVP. But as the time has gone on, we have seen different doctors and consultants with no results. All test show up clear.... In the back of my mind the last while has been the HVP vaccine. As elsewhere when you mention HVP you do not get any help. I am more and more convinced that her symptoms can be linked to the vaccine but here in Ireland I can't find anywhere to turn to get help to test for vaccine injury if such a test exists? So thank you Melissa for the links again. I will contact them and hope for the best. I wish you all out there with injured daughters and sons all the best. One day soon I hope they will recognise all the pain our children have been put through.
Kind Regards
Claudia
22 June 2018 at 10:25 am
Dear Claudia,
Thank you for sharing your story. We’re so very sorry to hear about your daughter and can empathise with the difficulties you must all be dealing with. We hope that our links connect you to others in a similar situation so that you can find more support and hopefully, some answers that help.
Warm wishes
Miranda
17 October 2017 at 2:44 am
We are told that our 11 year boys will need vaccinations. I see specific mention of HPV but also a lot of vague mention of "vaccines" on this website. When you say "vaccines" are you referring to even the basic vaccines such as TDAP? The suggested shots at age 11 for boys is TDAP and HPV. I've decided NOT to have them get the HPV, but I'm not clear if their is negative feedback on TDAP. Can anyone offer feedback?
Thank you in advance, TL
19 October 2017 at 9:04 am
Hello TL
All drugs have the potential to cause adverse reactions including vaccines. As with all medicines we would recommend you research the topic so you can make an informed decision based on that research. You may find this Facebook group of use as a starting place to ask questions of other parents who support natural health and question standard medical treatments - https://www.facebook.com/groups/arnica/.
Warm Regards
Melissa
20 October 2017 at 1:31 am
Hi there,
I live in Vancouver/ Canada and just this year they introduced the HPV vaccine for Grade 6 boys in school. I said no on the form and my son is devasteded! Every single kid on his class got the shot but him. He thinks I don't know anything and now he has a chance to get cancer. That's what his classmates told him.
I don't know what to do.... It's hard to believe that nobody knows about the side effects of this vaccine. How come the Health care that it's very good here is encouraging this. I don't get it!
28 October 2017 at 11:30 am
I live in the U.K. and my 12 year old daughter had bad seizures within 7 days of having Hpv vaccine. She has had three so far which have occurred in her sleep. She has been admitted to hospital and been kept in for observation and tests. We only got the consultants report y'day after eeg, ecg, MRI etc. The HPV vaccine was mentioned twice in the report as a possible link and we are now bring referred to cardiology as they have found an irregular heartbeat on the ECG. My daughter was fit and healthy prior to this and never had a day off school. She now has frequent spells where she feels dizzy and has headaches. It is affecting her concentration in school. I can't leave her on her own to sleep as the seizures so far have all occurred in her sleep due to her blood pressure dipping and not enough blood getting to her brain. I have to sleep with her until we get to the bottom of it as I'm afraid to leave her - it's like going back to when she was a newborn. My husband and I are very concerned - just can't believe this vaccine is being given to young girls! We had no idea if these side effects and only becoming aware as we are now researching it. Our lives have changed so much in just the last 4 weeks since she received the vaccine. Please be aware of the possible dangers!
30 October 2017 at 4:45 pm
Hello, thanks for your comment. We’re sorry to hear your daughter has suffered so badly following being vaccinated against HPV.
The UK Association of HPV Vaccine Damaged Daughters is a good resource and place to get support from other parents whose children have suffered adverse reactions as a result of this vaccine https://www.facebook.com/AHVID.UK/.
We send our best wishes to you both and hope your daughter gets the help and support she needs and things improve for you all.
Warm Regards
Melissa
29 October 2017 at 3:44 pm
Is there any evidence that the vaccine causes sudden onset bilateral low frequency hearing loss.
31 October 2017 at 8:34 am
Hello Patty
There have been reports (23) of hearing loss associated with the HPV vaccine through the VAERS (Vaccine Adverse Event Reporting System - https://vaers.hhs.gov/data.html) system in the US. Bear in mind though that a report to the VAERS system does not mean the symptoms were caused by the vaccine. But, also a report to the VAERS does not mean the new medical condition is NOT causally associated with the vaccine either. VAERS is simply an ‘early warning’ system. It is up to the CDC and FDA to examine the reports to determine whether or not a safety signal exists.
I hope this answers your question and helps.
Warm Regards
Melissa
20 November 2017 at 10:35 pm
I am 19 years old, I received the HPV vaccine the summer before my sophomore year of high school at age 15. The next morning I woke up in excruciating pain, my whole body felt bruised, I was dizzy and couldn't breathe, I couldn't move, and I had the worst headache I have ever experienced. I was screaming for my parents in pain and they immediately called my doctor and she told them not to worry that I would be fine in a few hours. The symptoms never went away and a few months later I was diagnosed with mononucleosis. After recovering from mono the symptoms never went away, I was still experiencing headaches, chest pain, and random body aches. My pediatrician refused to believe that it was the vaccine, she would tell my mom that I was either stressed, depressed or just looking for attention. My mom took me to Children's Hospital Los Angeles and the doctor at first told my mother that I most likely had a brain tumor refusing to blame the vaccine. After the CT scan came back negative for a brain tumor she told my mother that I was most likely depressed and prescribed antidepressants. To this day I still have headaches, chest pains, body aches, dizziness, troubles sleeping, and have experienced kidney inflammation. I used to be a straight A student and play competitive sports now I'm lucky if I can get through 1 full hour of studying without taking a break because of my headaches.
21 November 2017 at 8:24 am
Hello Gabriella, we're so sorry to hear about your experiences and appreciate you sharing your story. We're very concerned about the way doctors are treating children who suffer adverse effects from the HPV vaccine and are campaigning to stop health authorities from saying vaccines are safe. We would also like to see headaches become a serious adverse event rather than minor as they are currently as many girls have long-term issues in the same way you have. Although UK based, you may find the Association of HPV vaccine damaged daughters (https://www.facebook.com/AHVID.UK/) and Time for Action (https://www.facebook.com/TimeForActionOnHPVVaccines/) useful resources to help and support you.
Wishing you well.
Warm Regards
Melissa
27 November 2017 at 12:22 am
My big eye opener was Bill Gates statement in which he noted that one of the ways how to control the population is by strategically administering vaccines. I started researching more. By opting out of vaccines it made me feel an outsider. GP advised to read Green Book so I would be informed about vaccines. In that book I didnt find cases of adverse side effects, study cases of vaccinated vs non vaccinated. It didnt contain any solid evidence or facts just bla bla bla. 2 years ago it was 5in 1 vaccines plus 2 other ones, now its 6 in 1 plus 3 other ones for babies. I received 3 vaccines in my childhood compared to what its now. I will not believe that by increasing vaccines peoples health is improved.
27 November 2017 at 4:26 pm
My daughter’s 3rd vaccination was on 11/26/2016. Almost a year later 9/2017, she started having seizures. They have run MRIs, EEGs, EKGs, CT scans, blood work, even a stay at a mental institution because they thought it might have been anxiety. She has been dropped from cheerleading and has to be homeschooled because of this. I need answers. Could this possibly be caused by this vaccine. I am at my whit’s end, please help me. This is my daughters Sr. Year of high school.
29 November 2017 at 10:45 pm
Hi Robynne, we're so sorry to hear of your daughter's illness and the pain and anxiety you must all be going through. The key is to see an integrative medicine practitioner in the US who specialises in environmental causes of illness including side effects of vaccines. We'll send you a private email as it would be good to know where you are based so we can point you in the right direction more effectively. We really wish you and your daughter the very best at this difficult time.
Warmly, Meleni
03 December 2017 at 7:33 am
My daughter got this vaccination and then developed POTS. I'm not an antivaxer, I understand the importance of vaccines whole-heartedly, but I knew there was initially some concerns about this vaccine so I wasn't sure about it. But her doctor took a very hard stance on it and treated it like it was practically a requirement to do it while we were getting her boosters I really felt very pressured and didn't realize it wasn't mandatory .
My daughter started fainting repeatedly in school and by the end of the school year she was diagnosed with POTS so severe we have to do online school now. Some days she can't get out of bed. I was told it was likely due to puberty hormones and never realized it might have been due to the HPV vaccine.
11 December 2017 at 8:41 am
Hello TT
We're very sorry to hear of your daughter's illness and the way in which you've been treated by your Doctor.
We're hearing more and more stories of young girls suffering serious illness following HPV vaccine. The attitude of health authorities and doctors to these reports is one of the reasons we launched our petition [https://www.change.org/p/centers-for-disease-control-stop-health-authorities-claiming-that-vaccines-are-safe calling on health authorities] to stop health authorities from claiming vaccines are safe and conduct proper safety studies. All parents need to be given as much information as possible so they can make an informed decision about the potential impact of vaccines.
We hope your daughter's health improves in time and wish you well.
Warm Regards
Melissa
06 December 2017 at 2:47 am
After reading these comments, I would not put my family through these vaccines. I would trust the experience of people who has taken them from people who have not and said they are safe. Second of all, If I do not feel safe taking these, why would I let my family take them. Third if I encourage my kids to take these vaccinations, then I as a guardian should too.
09 January 2019 at 8:12 am
I received my first HPV Gardasil shot when I was 13. Before I had this shot, I had energy, bubbly, no pain, did great in school. But after the shot, everything changed.
I’m 26 years old and I’m having to apply for disability because of what the vaccine did. I have constant fevers, chills, nausea, vomiting, severe joint pain and more. I can’t hold down a job because I miss too much work. My doctor told me that the vaccine messed up my autoimmune system. I’m always sick. Most days I’m unable to get out of bed most days when I wake up. My morning always starts with intense anxiety and pain all over which usually makes me sick.
I also have Stage 4 endometriosis which was probably also caused by the vaccine but we don’t know for sure. I’ve had two surgeries for my endo in the past 4 years. I really wish I never got those vaccines. I’m thankful my sisters didn’t either!
10 January 2019 at 11:04 pm
Dear Hannah
Thanks so much for sharing your experience. We can only imagine how tough it's been for you and what life must be like every day. We send you our very best wishes and hope that you find a functional or integrative medicine practitioner to support your health journey. We also hope you've managed to reach out to support groups like the UK Association for Vaccine Injured Daughters (https://sanevax.org/uk-association-of-hpv-vaccine-injured-daughters-ahvid) to link up with others who find themselves in similar circumstances.
Warm wishes
Meleni
11 February 2019 at 4:06 pm
How long does it take for some symptom to come on in boys.
12 February 2019 at 10:37 am
Hello Shirley
It's very difficult to give a definitive answer as everyone is different. We would suggest you contact an organisation such as the UK Association for Vaccine Injured Daughters(https://sanevax.org/uk-association-of-hpv-vaccine-injured-daughters-ahvid) who also support parents of boys as well as girls.
We wish you well.
Warm wishes
Melissa
18 February 2019 at 1:35 pm
Thank you for all this info. My daughter's school is doing this vaccine in this week, she is 10. My father also has Lupus. I was not going to let them give this injection to her to begin with and after everything I read here I geel confident that I am making the right decission. Thank you all, this has confiemed my own conviction.
11 August 2021 at 1:00 am
I've just stumbled upon this and I wanted to ask if this could also be linked to Endometriosis?
21 September 2021 at 12:21 pm
I have the same question but haven't found any research online. I had the vaccine when I was at school and have since found out I have endometriosis.I feel like there is a link but not sure how to find out.
Your voice counts
We welcome your comments and are very interested in your point of view, but we ask that you keep them relevant to the article, that they be civil and without commercial links. All comments are moderated prior to being published. We reserve the right to edit or not publish comments that we consider abusive or offensive.
There is extra content here from a third party provider. You will be unable to see this content unless you agree to allow Content Cookies. Cookie Preferences