Content Sections
A European Commission draft Recommendation is soon to be set in stone and could drastically limit availability of food supplements available online.
If you want to skip the detail on the background to this draft Commission Recommendation, CLICK HERE for the campaign actions. People power really matters, please share widely!
What in the EU is going on?
The half a billion people living in the current 28, soon to be 27, member states of the European Union (EU) represent one of the world’s largest marketplaces. As the EU’s legislative vice around natural products selling through conventional retail outlets strengthens and more products are lost, more and more consumers are relying on e-commerce to get the products they rely on and sometimes can’t live without - literally.
Aside from this, there is a general and major trend towards the greater use of e-commerce, with the EU set for “impressive e-commerce growth” in the coming years.
People who regularly purchase health foods and natural health products are by and large those who take greatest responsibility for their health. Because of this, these same people – including supporters and followers of ANH – also pose the least burden on society when it comes to degenerative and chronic diseases. Why should they be penalised because of inappropriate, disproportionate actions by over-zealous national authorities spurred on by a badly constructed Commission Recommendation?
When it comes to regulations affecting natural health products in the EU, the water in the saucepan is at an ever greater risk of ‘boiling the frog’. We warned of this over 15 years ago and have worked to offset, delay or modify the application — and especially the over-zealous application — of these laws ever since.
e-Commerce crackdown: from ‘soft’ to ‘hard’ law
The latest European Commission (EC) draft recommendation seeks cooperation from EU member states over implementation of laws affecting natural health products. But if it isn’t to unnecessarily limit freedom of choice and omits to reign in the very small number of real ‘cowboy’ operators that do pose a risk to public health, it is in urgent need of adjustment.
As an EC recommendation, it’s an example of ‘soft law', so it isn’t legally enforceable in itself. But the ‘hard laws’ it points to are most certainly enforceable.
The draft recommendation focuses on 4 ingredients in food supplements, namely agmatine, Acacia rigidula (a key source of phenylethylamine or PEA), horny goatweed and Hoodia, all of which pose little or no risk to human health when used appropriately in food supplements. Yet these ingredients when added to products have clear benefits (see infographic below).
Among their sins is the fact the ingredients don’t have a proven history of sale in the EU prior to 15th May 1997 (the implementation date of base EU Novel Food Regulation) and they, in turn, have not been authorised as novel foods following a safety evaluation by the European Food Safety Authority (EFSA).
This date, now over two decades in the past, is fast becoming nigh on impossible to abide by given accountancy and sales records are rarely stored for more than 6 or 7 years.

Fig 1. Infographic summarising EC draft recommendation on food supplement e-commerce.
When democracy is cast aside
EC soft laws have been far outstripping the passage of legally-binding hard laws, i.e. directives and regulations, in recent years (Fig 2). In this case, soft law in the form of a Commission Recommendation, that doesn’t need to go through formal parliamentary scrutiny, is being used not only to increase cooperation between authorities in the different EU member states, but also to bring in altogether new requirements, such as the suggestion for quality seals for e-commerce operators, along the lines of those used by online pharmacies. We think this is a disproportionate requirement that places a burden on online sellers of foods that greatly exceeds that for ‘brick and mortar’ or high street retailers.
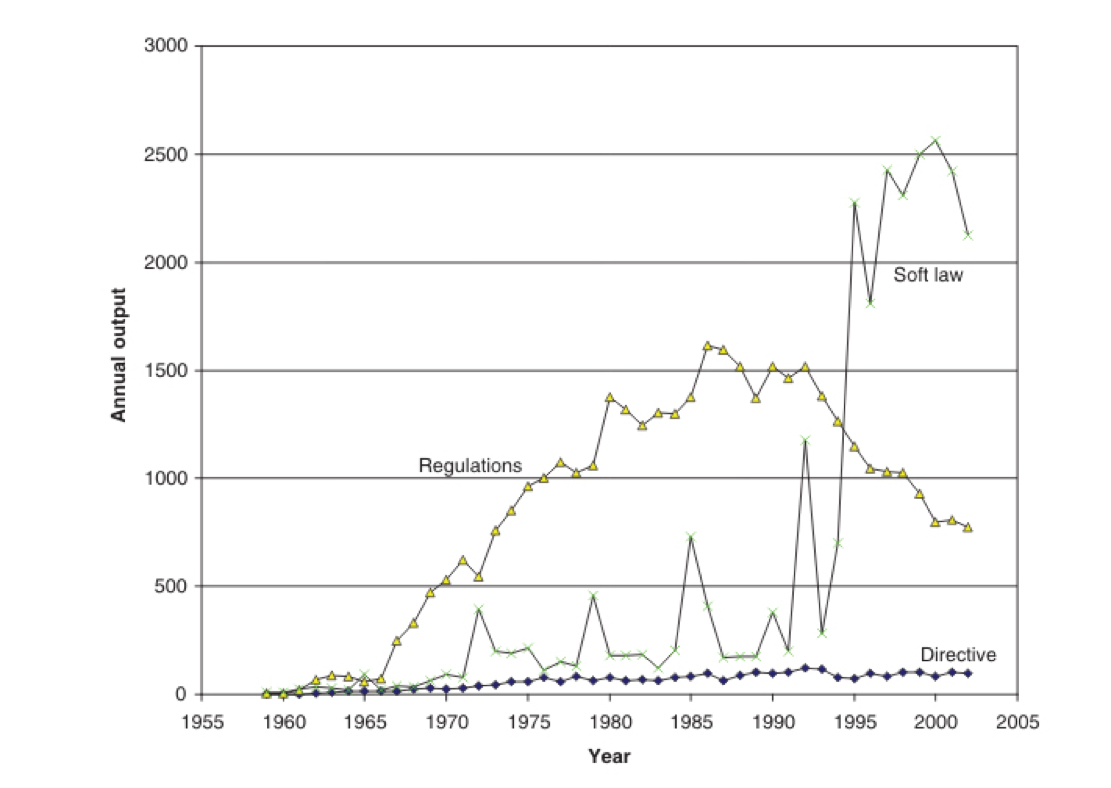
Figure 2. Number of Directives, Regulations and soft laws issued since the EU’s inception (Source: Christensen, 2010)
The European Parliament, in its 2007 resolution on the institutional and legal implications of the use of “soft law” instruments revealed its concerns over the possibility of soft law bringing "confusion and uncertainty" to a field (preamble, paragraph N). That's exactly our concern - and it works contrary to the treaty obligations of the European Commission's role as executive to improve the functioning of the single market and protecting the fundamental rights and freedoms of its citizens. Another concern raised in the Parliament's 2007 resolution related to the "democratic deficit". The resolution states “it is regrettable that the involvement of Parliament and the Court of Justice therein is very weak” (preamble, paragraph P).
The additional requirements linked to the Recommendation were proposed in the related EC Action 23 to which we drew attention last week, that is proposing that online traders be required to display quality seals that are on par with those required for “legally operating online pharmacies”.
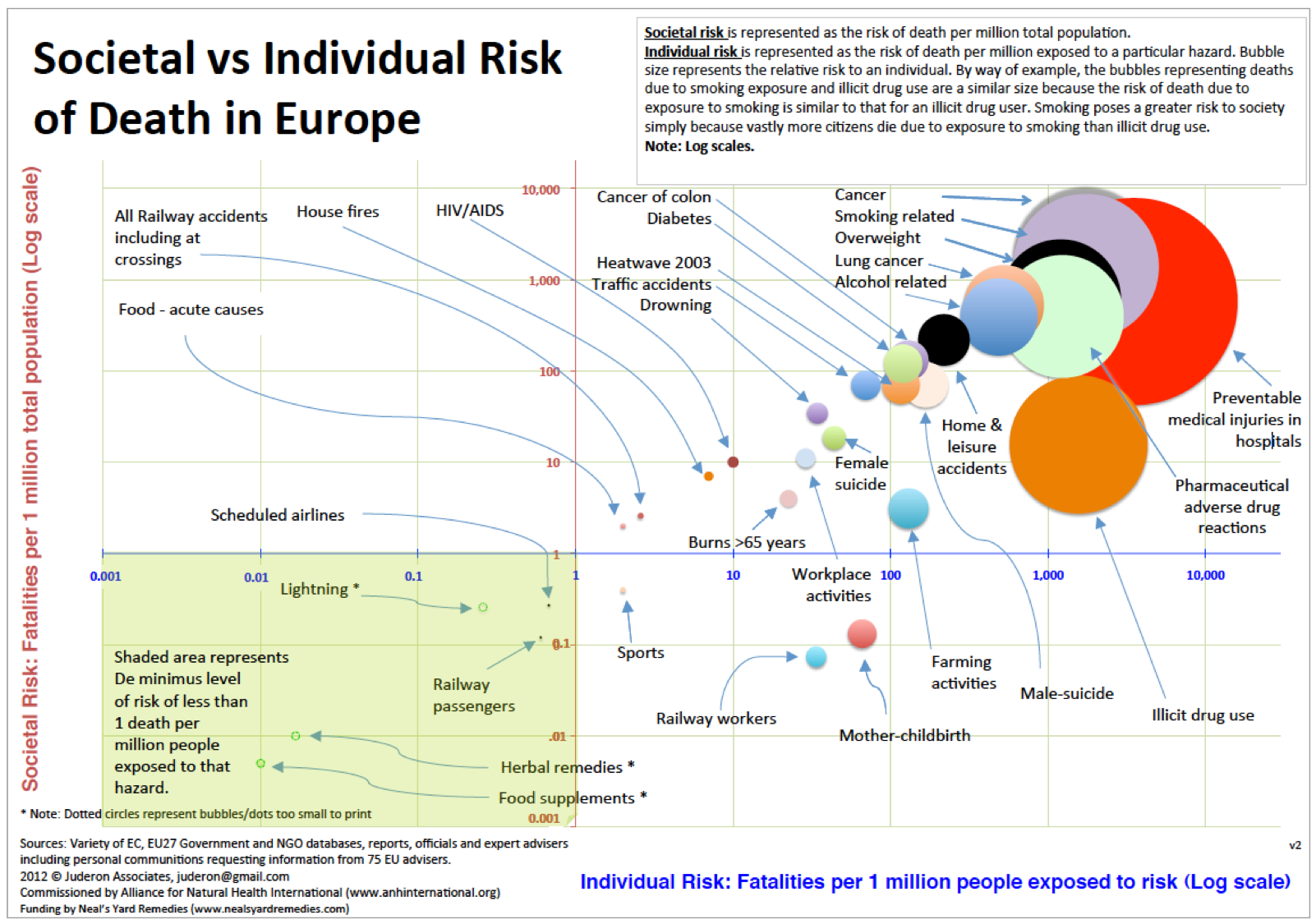
We argue this requirement is an excessive burden for products that are legally sold as food supplements, which are legally a sub-category of food not medicines and have a safety record that is considerably greater than foods, and orders of magnitude greater than the licensed medicines sold by pharmacies or used in hospitals (Fig. 3).
Fig 3. Relative risks bubble chart: societal vs individual risk of death in the EU, based on official figures. (Source: ANH-Intl)
The campaign
We believe it’s essential to modify this Commission Recommendation while it is still in draft form. This requires urgent action by EU citizens as well as their elected representatives in Brussels, namely Members of the European Parliament (MEPs). We think this recommendation represents the tip of an iceberg and if implemented in its current form, and then extended as planned, it could dramatically strip EU citizens of their freedom of choice over food supplement products that have clear health benefits and pose little or no significant public health risk.
We have written to the European Commission today, we are engaging with MEPs and we are actively working with collaborators in other parts of the EU so that they can take the message, in their national languages, to the public, to their elected representatives and to the European Commission itself.
Our key messages are as follows:
- Prevent unnecessary confusion and uncertainty through the misuse of 'soft law' that contravenes the European Parliament's resolution dated 4 September 2007 (P6_TA-PROV(2007)0366) on use of 'soft law' instruments
- Remove the focus in the current draft Commission Recommendation on novel food ingredients that pose no genuine or significant risk to public health
- Redirect the focus from ingredients within products to commercial products themselves
- Focus in particularly on products that have been demonstrated to pose a genuine and significant public health risk, such as those identified via the Rapid Alert System for Food and Feed (the RASFF system)
- Recognition must be given to the extensive EU case law established by the Court of Justice of the European Union (CJEU) that relates to the approximation of laws affecting food supplements products, as compared with the presence (regardless of dosage) of the individual ingredients within them
- Seek an amendment to the Novel Food Regulation that revises the arbitrary cut-off date of 15 May 1997 given sales and accountancy records going back this far are generally no longer available and the Novel Food Regulation now acts as a disproportionate and protectionist barrier to freedom of choice by EU citizens
- Remove the requirement for online sellers to have quality seals (as suggested in EC Action 23) as this is disproportionate and places a burden on online sellers that exceeds those of conventional offline retail outlets
- Given limited resources, enforcement actions against e-commercial enterprises taken by EU member states should be prioritised according to the genuine public health risk posed by the commercial products
- The draft recommendation should be amended taking into account the above concerns.
We need your help NOW!
Please write to the Commissioner for Health & Food Safety, Vytenis Andriukaitis and tell him that you think the draft Recommendation (SANTE/7036/2017) needs amending.
The reasons are explained in our campaign poster.
Email: [email protected]
Postal address: European Commission, Rue de la Loi / Wetstraat 200, 1049 Brussels, Belgium
Please also take the above points and ask your MEP to tell the European Commission to amend urgently its Draft Commission Recommendation (SANTE/7036/2017) “on a coordinated control plan on the official control of certain foods marketed through the Internet”.
Find your MEP here.
Click here to download an example letter that you can edit and personalise, as politicians respond far better to personalised information that is made unique.
Please share this article, campaign message and poster (click here to download) widely to your family, friends and contacts across the EU. Print off the campaign poster and distribute, post in shop windows and anywhere else you can think of!
Follow us on Facebook, Twitter (@anhcampaign) and Instagram (@anhintl) to keep up-to-date with the campaign and share campaign messages, memes and infographics.
Please click on poster image below to open PDF.








Comments
your voice counts
26 July 2017 at 10:06 pm
Please, go together with one of the petition sites, so that it is easier for any user to post his opinion to the addressee. Thanx!
27 July 2017 at 1:51 pm
Hello Bernd, thanks for your comment. In this instance a petition would not be appropriate. A campaign of this type needs personal letters to be sent to the Commission and MEPs as it is far more effective.
We are currently working on an automated system that will make it much easier for people to express their opinions in future.
Warm Regards
Melissa
11 October 2017 at 8:28 pm
Hi, Melissa
I think you're not exactly right.
The advantage of a major e-petition is that it can harness the power of social media - multiple re-tweets, intertest picked up by independent bloggers - to gain much wider support than individual activist websites can manage. These things, if well-managed, also have a greater impact on general public perception and democratic influence.
And, of course, one can hang letter-writing campaigns, requests for funding, and news updates onto the petition updates.
By contrast, it's quite difficult to get masses of people to write personalised letters, which they need to put some thought into. And find out who to send them to. So, we don't, or don't have time. It doesn't get done. Goes flat.
On top of this, the average MEP's office just doesn't read the letters.
Staff (or automatons) spot the first keyword, send a standard reply which can be completely off-beam, add one to the count of things 'solved' and move on. Pointless.
One of my own MEPs was quite dismissive - not interested in the topic, not interested in my specific comments, and decisions on such things are made by an internal Party committee (in league with corporates) - so MEPs are just following ze orders.
Personally, I regard that as tantamount to a corrupt system - but whatever my view, it gets us no-where.
E-petitions and harnessing social media are the way to go.
11 October 2017 at 8:31 pm
typo! *intertest = interest
16 October 2017 at 9:59 am
Thanks for your feed back Bill, it's always appreciated.
Warm Regards
Melissa
27 July 2017 at 8:59 am
This move by the EU would just fuel the goals of Big Pharma to take away human rights of freedom of choice over their own health choices. Many people choose to use supplements to support their health as a preventative to avoid the onset of major conditions/diseases. Many supplements are more effective than drugs and significantly less damaging, which is a major problem for Big Pharma. Please look at how ingredients work synergistically rather than individual ingredients within a supplement. I am a qualified nutritional therapist and I see the massive value every day in the availability of supplements online via practitioner recommended sites. Please consider the basic human right to make our own choices about our health.
27 July 2017 at 9:15 am
Hi Diane. This is exactly why we need to come together and fight this Recommendation. So we can maintain freedom of choice when it comes to our healthcare.
Thanks for taking the time to comment.
Warm regards
Melissa
27 July 2017 at 10:11 am
Many of my clients would be thrown back onto having powerful psychotropic drug with terrible side effects were their supplements to become unavailable. This and the vaccine issue points to an increasing nazification of our health services.
10 October 2017 at 10:17 pm
Should I still write MEP's or is it already to late?
11 October 2017 at 4:40 pm
Yes please. There is still time to write to your MEP about this issue.
Warm Regards
Melissa
Your voice counts
We welcome your comments and are very interested in your point of view, but we ask that you keep them relevant to the article, that they be civil and without commercial links. All comments are moderated prior to being published. We reserve the right to edit or not publish comments that we consider abusive or offensive.
There is extra content here from a third party provider. You will be unable to see this content unless you agree to allow Content Cookies. Cookie Preferences