Content Sections
- ● Why is there a need to regulate?
- ● What are the main challenges brought by regulation?
- ● What is the EU Definition of a medicine?
- ● About EU 'ring fences' and the EU Food-medicine borderline
- ● Medicinal Law's ‘loaded gun’
- ● How can practitioners stay safely within 'food' territory?
- ● EU Food categories particularly relevant to practitioners
- ● Plant-based natural health products
- ● EU Nutrition and Health Claims Regulation
- ● Are your food and supplement claims ‘authorised’ or ‘non-authorised’? Finding your way around the EU Register of Nutrition and Health Claims.
- ● Additional points for Practitioners
- ● ANH’s articles on the potential impact of Brexit on the regulation of natural health in the UK
The specific laws that affect them will often be a mixture of national laws, some of which may be based closely on EU laws (notably if they were based on EU Directives), and of EU laws. EU laws come in multiple forms; Directives need to be transposed into each of the national legislative systems, whereas EU Regulations apply as they stand in all EU member states. There are also EU Decisions or Recommendations that may be relevant.
The following discussion relates mainly to those practitioners that are involved with provision of nutritional, food supplement or herbal products, as this affects a large number of practitioners and EU regulations have had a great impact on this group.
Following is a diagram summarising the key EU regulations affecting the sale of food supplements in the EU.

The most influential pieces of law include European general food law, the controversial Nutrition and Health Claims Regulation, EU medicines law, EU food supplements law, herbal medicines law, the Novel Food Regulation and and the Food for Consumer Information Regulation (FICR). They all work together so creating a complex regulatory framework that increasingly impinges on the practices of naturopathy, nutritional therapy, herbalism and related disciplines.
Why is there a need to regulate?
- From the regulator's perspective: to ensure a "High level of consumer protection" and "Functioning of the internal market"
- From the practitioner's perspective: To ensure safety and professionalism
What are the main challenges brought by regulation?
- Restrictions on product availability
- Restrictions on freedom of speech/communication
- Restrictions on using scientific evidence for support
What is the EU Definition of a medicine?
- A product is medicinal by presentation (Presented to treat or prevent disease, or to address a diagnosis)
- A product is medicinal by function (It corrects, modifies, restores physiological functions, or it has a pharmacological, immunological or metabolic action)
- A product is "clearly" not a food, food supplement or cosmetic
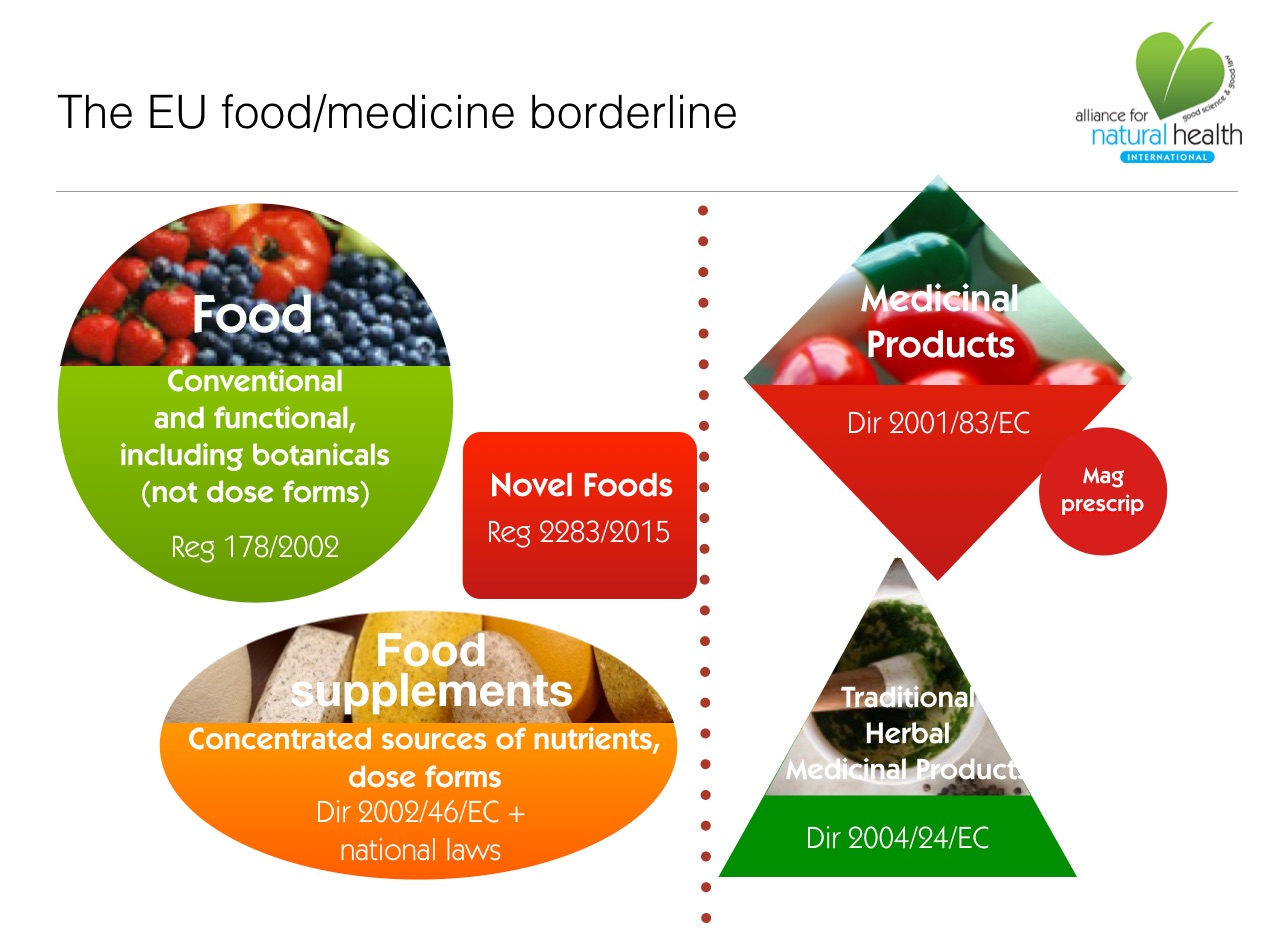
About EU 'ring fences' and the EU Food-medicine borderline
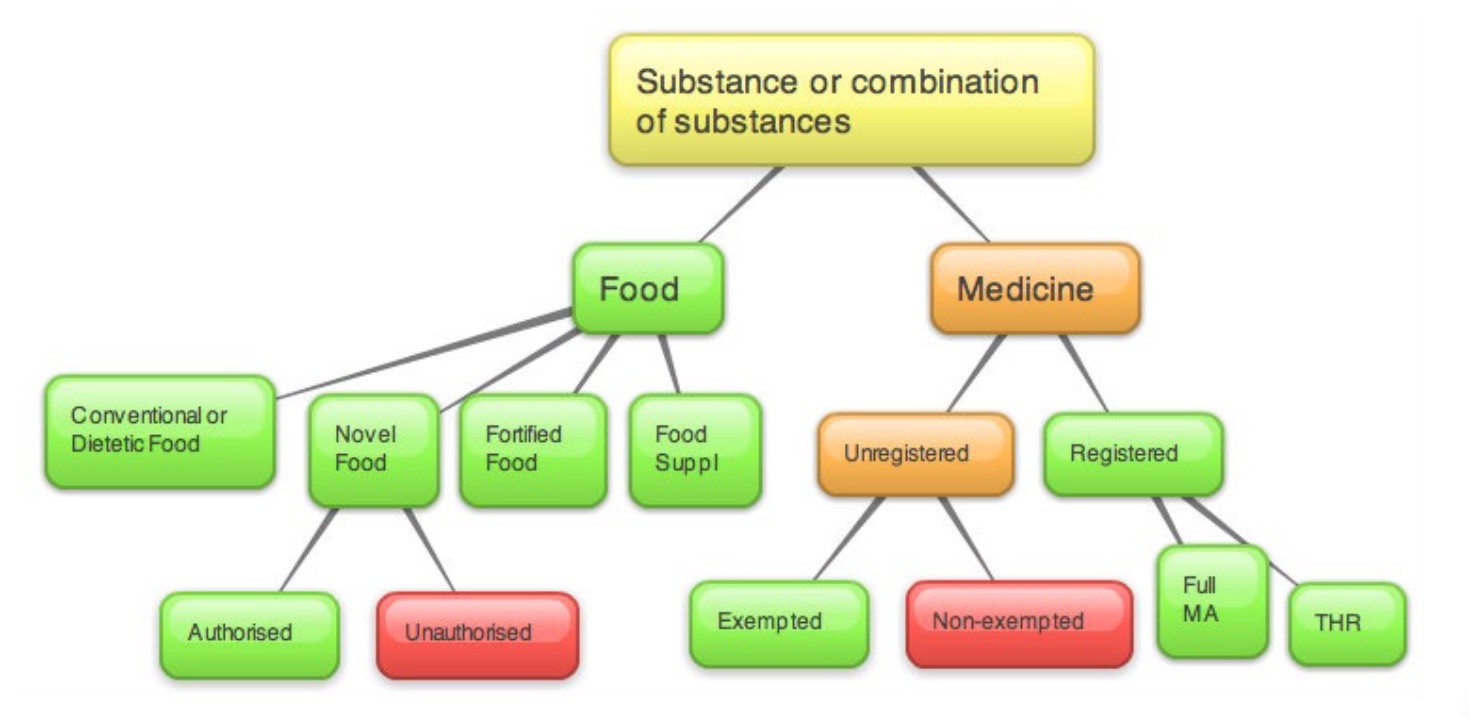
A practitioner's presentation of a product he or she is using can turn it from a simple food supplement to an illegal, unlicensed medicine incurring the wrath of health authorities, national drug regulators and even authorities setting out marketing and advertising guidelines, such as the Advertising Standards Authority in the UK! It's important for practitioners to know that, with the exception of those medically trained, they cannot use or recommend ‘unregistered medicines’ (or ‘unauthorised novel foods'). Unfortunately, regulation—most imported directly or indirectly from the EU—prevents practitioners from prescribing or recommending natural products that are different to those available to lay members of the public.
Life isn’t made any easier by the fact that drug regulators makes medicinal determinations on a case by case basis, being guided by case law established in the European Court of Justice. An absolute definite for a medicinal determination is a product that falls foul of the first, ‘presentation limb’ of the definition of a medicine, as set out in the EU Human Medicinal Products Directive (HMPD; Directive 2001/83/ EC, as amended): “Any substance or combination of substances presented for treating or preventing disease in human beings...”.
Medicinal Law's ‘loaded gun’
But it’s falling foul of the second, ‘functional limb’ of the definition that makes things complicated: “Any substance or combination of substances which may be used in or administered to human beings either with a view to restoring, correcting or modifying physiological functions by exerting a pharmacological, immunological or metabolic action, or to making a medical diagnosis”. And that’s mainly because every food product or beverage consumed has those actions! It’s only the 7th sentence of the 7th recital (preamble) of the HMPD that saves us. And while this is open to wide interpretation, it states that products that are “clearly” foods or food supplements should fall outside the scope of EU medicines law. But because recitals have less weight than articles, and another article (2.2) states that medicinal law should always apply in cases of doubt, it becomes doubly important that a food supplement product, in terms of its presentation, activity, form and dosage, can be viewed clearly as such.
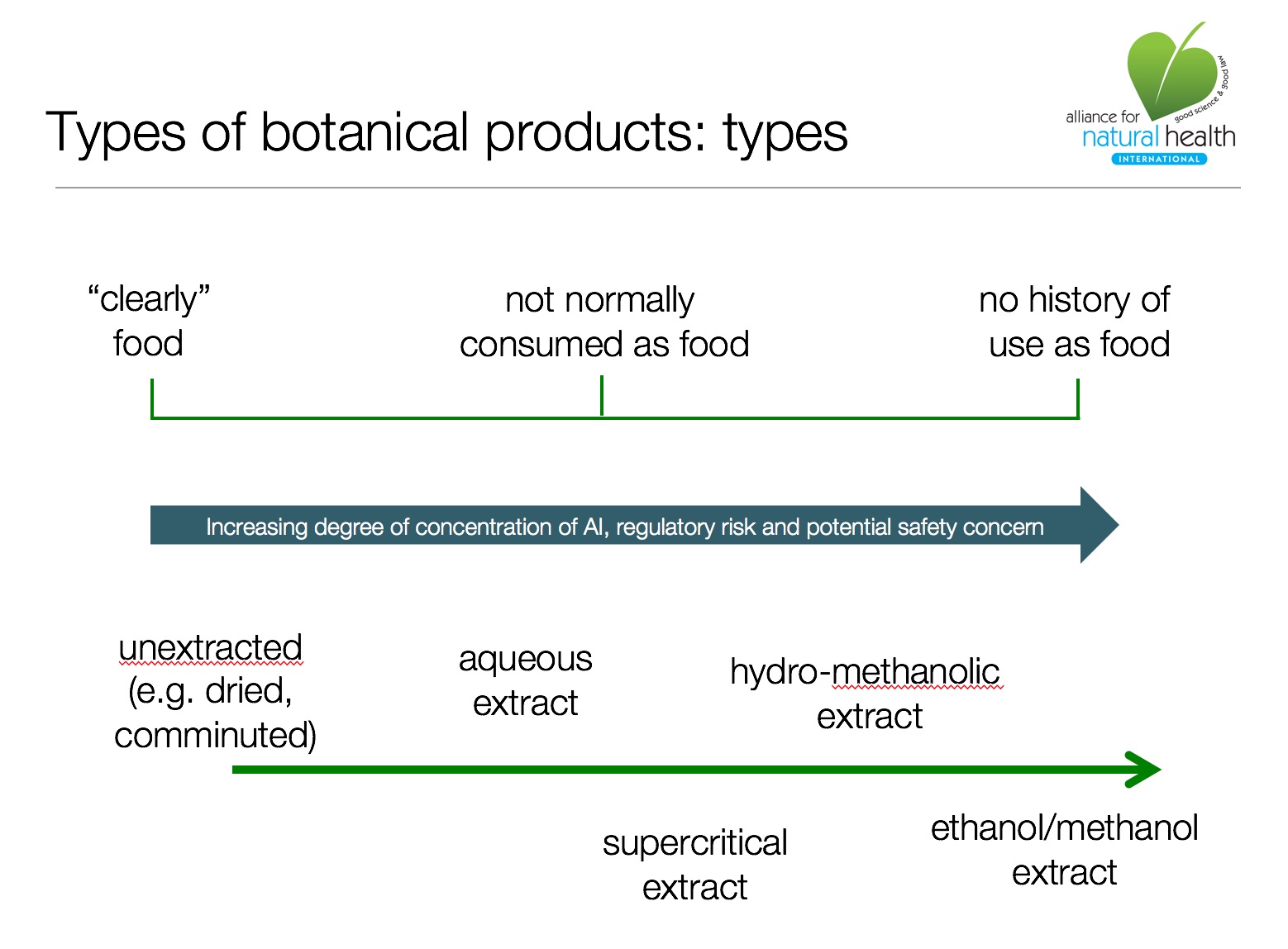
For products that include botanicals, this means that a history of food use, un-extracted or aqueous extracted forms, and lower inclusion amounts, tend to be key factors that support the food supplement status of a product. In contrast, highly concentrated ethanolic extracts, particularly of herbs or herbal parts that have not had a history of food use, are much more likely to trigger a medicinal determination by the HMPD.
How can practitioners stay safely within 'food' territory?
- Understand that there are two limbs to the definition of a medicine that govern both the presentation and function of a product. Be aware of the words you use and never present commercial food supplements a) for treatment/prevention of disease, b) correcting, modifying or restoring physiological functions, c) exerting pharmacological, immunological or metabolic functions, or d) being used for medical diagnosis
- Except where authorised health claims apply to commercial products, talk in generic terms about the nutrients themselves, rather than using brand names of commercial products when describing the action or effect on the body. Again, except for the very limited authorised Article 14.1a health claims, never associate commercial products with their influence on disease risk factors
- Stay up-to-date with current authorised and non-authorised health claims (EU Register), and only use authorised claims when referring to commercial products
- See our Practitioner Page on “Guidelines on what to say when advertising in the EU”, about phrasing the sentences on your websites and in your marketing materials to avoid making unauthorised medicinal claims
- Further recommendations are to be found on our practitioner leaflet
- Keep up to date with our work, and please support our campaigns, particularly when we urge specific actions to protect our access and use of natural products
EU Food categories particularly relevant to practitioners
- Foodstuff [Regulation (EC) 178/2002 / Food Safety Act 1990]
"...food (or "foodstuff") means any substance or product, whether processed, partially processed, intended to be, or reasonably expected to be ingested by humans..."
"Food shall not include... medicinal products within the meaning of Council Directive 65/65/EEC [now Directive 2001/83/EC]" - Food supplements [Food Supplement Directive (2002/46/EC) / Food Supplement Regulations 2005]
"foodstuffs the purpose of which is to supplement the normal diet and which are concentrated sources of nutrients or other substances with a nutritional or physiological effect, alone or in combination, marketed in dose form"
Chemical forms of vitamins and minerals must be on the Food Supplements Directive (FSD) positive list [Regulation (EC) No 1170/2009], i.e harmonised EU-wide. Natural sources are outside the scope of the positive list. For other ingredients, national rules apply.
Maximum Permitted Levels of vitamins and minerals: These are still intended to be harmonised EU-wide, with both maximum and minimum permitted levels. They are to be applied to food supplements and fortified foods. ANH has created a major blockage to this process, with MEP support on the basis of arguments set out in 2 peer-reviewed papers. The papers revealed major scientific weaknesses in the approaches being considered by the EC to harmonise EU-wide maximum vitamin and mineral doses in supplements. ANH-Intl subsequently commissioned TNO scientists in Holland to develop a new methodology that developed a scientifically robust approach that overcame these weaknesses. The method has now been published 'ahead of print' in the leading, peer-reviewed scientific journal Critical Reviews in Nutrition and Food Science. - Dietetic foods and supplements
The EC refer to these as "Dietetic Foods/Foods for specific groups", and they now replace the PARNUTS regulation:
"Dietetic foods, or foods for particular nutritional uses, are foods that, because of their special composition or manufacturing, are aimed to satisfy the special nutritional needs of specific groups of people such as:
Infants and young children
People undertaking energy-restricted diets to lose weight
People with specific medical conditions
People with gluten intolerance"
The New Regulation on Food for Specific Groups:
"The Regulation (EU) No 609/2013 of the European Parliament and the Council on food intended for infants and young children, food for special medical purposes, and total diet replacement for weight control ('Food for Specific Groups') was adopted on 12 June 2013. It will apply from 20 July 2016 and aims to protect specific vulnerable groups of consumers by regulating the content and marketing of these "special" food products offered to them. It also aims to increase legal clarity for business and to facilitate correct application of the rules" - Fortified foods
- Novel foods [Regulation (EC) No 2283/2015]
Pre-market authorisation of food or ingredient required if significant use cannot be demonstrated before May 1997 where: Primary molecular structure is new or intentionally modified; consists of or isolated from microorganisms, fungi or algae; consists of plants or isolated from plants or animals (except where traditionally propagated and history of safe use); It's a new production process which significantly changes composition or structure of food or ingredients. To establish whether a food or ingredient is novel: Is there evidence of significant EU usage prior to 15 May 1997?; See guidance for botanicals:- Novel Food Catalogue; Consult UK Food Standards Agency or Novel Food Working Group.
Plant-based natural health products
Relevant legislation:
- Traditional Herbal Medicinal Products Directive (THMPD; Directive 2004/24/EC)
- Human Medicinal Products Directive (HMPD; Directive 2001/83/EC, as amended)
- Food Supplements Directive (FSD; Directive 2002/46/EC, as amended)
- Novel Foods Regulation (NFR; Regulation 2283/2015, as amended)
- Nutrition and Health Claims Regulation (NHCR; Regulation 1924/2006, as amended)
- See also European Medicines Agency information on Herbal medicinal products and EFSA Compendium of Botanicals.
Also known as botanicals, under EU legislation, these can be foods, food supplements, herbal medicines of pharmaceutical medicines. The extremely broad scope and definition of a medicine (see above) allows regulators to classify many botanicals in supplements and functional cosmetics as medicines, unless they are 'clearly' foods, supplements or cosmetics. In cases of doubt, medicines law reigns supreme. Botanical food supplements are regulated by national rules in individual Member States. Increasing numbers of botanicals are being classified as novel foods, as above, for example where a novel preparation method is introduced.
Manufactured herbal medicines can be placed on the EU market as medicines either with full marketing authorisation or traditional use authorisation under the terms of the HMPD, or with traditional herbal registration (THR) under the terms of the THMPD. This THMPD is a simplified registration scheme which replaces the requirement for efficacy via human trials with evidence of long-standing safe use.
The THMPD suits phytopharmaceutical products, many of which are single-herb or limited combinations extracted using alcohol or acetone, stabilised in a base containing artificial polymers and preservatives. It locks out a very large number of products associated with non-European traditions, such as Ayurveda, Tibetan, traditional Chinese medicines (TCM) and Amazonian systems. Should such products be also squeezed out of the food supplements regime, they 'fall between two stools' of EU food and medicine law, and are consequentially illegal.
THMPD is also restricted to products intended for minor, self-limiting conditions, and which are used without practitioner oversight. The registration process is costly and cumbersome, being unaccomodating for polyherbal products.
National regulators are increasingly assuming that the only route to market for botanical products is for them to obtain a THR, and they are ignoring the more lenient food supplement route. In some cases, botanicals are able to be sold as food supplements in one Member State, but are classified as a medicine or novel food in another. To prevent this, pressure needs to be applied for 'mutual recognition'.
EU Nutrition and Health Claims Regulation
Relevant legislation:
- Nutrition and Health Claims Regulation (NHCR; Regulation 1924/2006, as amended)
What are the stated objectives?
- High level of consumer protection
- Avoid false or misleading claims
- Free movement of goods/ functioning of the single market
NHCR works as a centralised pre-market claim approval i.e. all claims are banned except those specifically allowed. There are separate controls of nutrition profiles, nutrition claims and health claims. NHCR applies to "... nutrition and health claims made in commercial communications, whether in the labelling, presentation or advertising of foods to be delivered as such to the final consumer..." [Article 1(2)]. Practitioners have to decide whether any claims they are making are within or outside the scope of the NHCR. Whether or not the claim is made in a commercial context is key. Up until now, nutrition and health claims made to the natural products trade, to health professionals, practitioners, practitioner associations, etc. are outside the scope of the NHCR on the condition that they do not influence the final consumer! But this may change.
Nutrition claim definition (Article 2.2(4)):
'Nutrition claim' means any claim which states, suggests or implies that a food has particular beneficial nutritional properties due to:
(a) the energy (calorific value) it
(i) provides;
(ii) provides at a reduced or increased rate; or
(iii) does not provide; and/or
(b) the nutrients or other substances it
(i) contains;
(ii) contains in reduced or increased proportions; or
(iii) does not contain;
Health claim definition (Article 2.2(5) and (6)):
- 'Health claim' means any claim that states, suggests or implies that a relationship exists between a food category, a food or one of its constituents and health
- 'Reduction of disease risk claim' means any health claim that states, suggests or implies that the consumption of a food category, a food or one of its constituents significantly reduces a risk factor in the development of a human disease
Are your food and supplement claims ‘authorised’ or ‘non-authorised’? Finding your way around the EU Register of Nutrition and Health Claims.
Since the end of 2012, many ‘general function’ (Article 13(1)) health claims about the benefits of foods and nutrients have been illegal in Europe. These are claims on labels, but also claims in any other medium, including — in law — the spoken word. Of all the claims now evaluated by the European Food Safety Authority (EFSA http://www.efsa.europa.eu), the only ones now permitted (most evaluated claims have been rejected due to inadequate scientific substantiation) are those that have been specifically authorised by the European Commission (EC). This has been done via Commission Regulation (EU) 432/2012 http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2012:136:0001:0040:en:PDF. (Reasons given by EFSA for lack of scientific substantiation are: A causative relationship is very difficult to prove; Human studies on healthy populations are not available or are insufficient and inconsistent; Observation and epidemiological evidence cannot be used as primary evidence; Characterisation of food/constituent in study is inadequate).
Whilst practitioners will still be able to speak and write about the health benefits of food and food constituents generically, they will be technically breaking the law if they speak or write about a specific commercial product in association with a so-called ‘non-authorised’ health claim.
So how will practitioners know which claims are ‘authorised’ and which are ‘non-authorised’?
The EC has established a website that is referred to as the ‘EU Register of Nutrition and Health Claims’: http://ec.europa.eu/nuhclaims. This includes nutrition claims as well as health claims, the latter being in one of four categories: general function (Article 13(1)), emerging science (Article 13(5)), disease risk reduction (Article 14(1)a) and children’s health claims (Article 14(1)b). Authorised claims are very limited in Article 13(5) (e.g. water-soluble tomato concentrate) and 14(1)a claims over only a narrow range of nutrients (e.g. stanols/sterols and oat beta glucan for cholesterol reduction, xylitol in chewing gum). In practice, this means that general function claims are by far the most numerous and useful group for practitioners. An example of an authorised general function claim is: ‘Magnesium contributes to normal muscle function, whilst an example of an non-authorised claim is: ‘Coenzyme Q10 maintains a healthy heart’.
How to use the EU Register of Nutrition and Health Claims
Once on the Register’s homepage http://ec.europa.eu/nuhclaims, click on the blue ‘EU Register of Nutrition and Health Claims’ tab. You will then be directed to a small ‘Terms and Conditions’ box, which you are required to read before proceeding. This summarises the law with regard to health claims, so that the reader is in no doubt about what is and is not permitted.
You may then find it helpful to use the register in the following way:
- The ‘Claim status’box: Click on ‘Authorised’ or ‘Non-authorised’ depending on whether you wish to see a list of permitted or non permitted health claims. Leaving the word ‘Status’ in this box will bring up both Authorised and Non-authorised lists according to the other variables entered in the other boxes. The ‘Authorised’ claim is all anyone is permitted to say or write about that nutrient or substance. Anything else is illegal, unless it is a botanical or probiotic that has yet to be evaluated fully by EFSA.
- The ‘Type of claim’ box: Click on ‘Art.13(1)’. This will bring up the ‘general function’ claims, which will be of the most relevance to practitioners.
- The ‘Legislation’ box: Entering ‘Commission Regulation (EU) 432/2012 of 16/05/2012’ in this box will also bring up the 222 authorised Art.13(1) general function claims.
- The ‘Search’ box: You may use this box to search for individual nutrients or substances, but you may need to check both in the ‘Authorised’ and ‘Non-authorised’ lists. If something does not appear on the ‘Non-authorised’ list it doesn’t follow that it will be on the ‘Authorised’ list. The majority of ‘botanical claims’ have yet to be evaluated (the vast majority of claims relating to herbs, many phytonutrients, mushrooms, algae and bacteria, notably probiotics) and do not yet feature on the EU Register. If it has yet to be fully evaluated, transitional measures will apply, which will permit the claim to be used under transitional measures, assuming the claim was already used before 1 July 2007.
We suggest that practitioners familiarise themselves with this EU Register, and with the authorised and non-authorised general function health claims, especially if you are going to be making claims about products in an environment which may result in your claim being brought to a regulator’s attention. It is obviously particularly important that websites and printed marketing materials only use authorised and transitional measures health claims for commercial products. However, we also strongly recommend that product recommendations made to clients/patients should also be free of non-authorised health claims.
Additional points for Practitioners
- Nutrition & health claims "shall not state, suggest or imply that a balanced and varied diet cannot provide appropriate quantities of nutrients in general". (Article 3(d))
- "Nutrition and health claims shall be based on and substantiated by generally accepted scientific data". (Article 6(1))
- "A food business operatormaking a nutrition or health claim shall justify the use of the claim" . (Article 6(2))
- "The competent authorities of the Member States may request that a food business operatoror a person placing a product on the market to produce all relevant elements and data establishing compliance with this Regulation". (Article 6(3))
ANH’s articles on the potential impact of Brexit on the regulation of natural health in the UK
http://anhinternational.org/2016/07/06/eu-distorted-dozen/


