Robert Verkerk PhD
Executive and scientific director, ANH-Intl
While you should always be cautious about extrapolating results from rodent studies to humans, it’s noteworthy that a group of Canadian researchers have discovered that high-dose folic acid supplements given to rats can have tumour-promoting effects (PLoS ONE, 2014: 9(1); e84635). They found that supplemented rats suffered higher rates of mammary tumours when malignancies had been chemically initiated in the cancer-susceptible Sprague-Dawley strain.

A new question mark over folic acid?
This is not the first study suggesting a potential link between high-dose folic acid and cancer. In 2009, a study by Norwegian researchers published in JAMA showed increased incidence of lung cancers among those supplementing with folic acid. Other evidence has also suggested prostate and lung cancer incidence might be increased.
This rather un-straightforward relationship between folic acid and cancer may not be so surprising. Let’s look at this in a little more depth.

A double-edged sword
For most vitamins and other essential nutrients, evolution has resulted in a top-class balancing act that uses nutrients in specific forms and amounts in very specific ways to deliver specific functions. In the case of folate, the vitamin group to which folic acid belongs, the functions are both huge in number and fundamental to animal life. One of its most important roles is in building and repairing DNA and consequently cells, hence the key role of folate in growth and development – and its ability to protect against neural tube defects in developing foetuses.
This DNA and cell building and repair function also makes folate indispensible for those areas of the body with high cellular turnover. The immune system and gastrointestinal tract immediately spring to mind.
Among its many other functions, the vitamin acts as a vital cofactor in the production of energy in cells, the prevention of cognitive decline, homocysteine metabolism and endothelial function. This is why folate protects against such things as fatigue, Alzheimer’s disease and certain forms of heart disease. Therefore, be folate-deficient at your own peril.
But folate can also have a darker side. What some of the recent work on folate appears to suggest is that while folate can fuel the very system – the immune system – that prevents tumour initiation, it may also be able to fuel the proliferation of cancers that have already been initiated. And balancing these counteracting processes is quite some feat.
Balance of evidence
So, where does the overall evidence of the benefits or risks of high-dose folic acid intake stand, given that the results of studies are, unsurprisingly, mixed?
The most common tool used to evaluate the balance of evidence is, of course, the meta-analysis (study of studies). The most comprehensive meta-analysis to date was published last year in The Lancet journal (2013; 381 (9871): 1029-1036). After examining data on 50,000 subjects from 13 trials, the researchers from Oxford University concluded that there was no statistically significantly increased – or decreased – risk of colorectal, lung, breast or prostate cancer among supplemented groups. What they did find, however, was somewhat disturbing: a weak, statistically non-significant 6% greater cancer risk overall among those supplementing with the vitamin, compared with non-supplementing subjects. But, interestingly, those who took the highest doses didn’t appear to be at any greater risk. Nor was there any suggestion that those who supplemented for longer periods were more likely to get cancer.
Much of the justification for all the intervention studies using folic acid has come from epidemiological evidence, i.e. the retrospective study of human populations. Here, we see a strong trend for reduced risk of colorectal cancer and, increasingly, pancreatic cancer among those with higher dietary intakes of folate. And, as the science is also showing ever more clearly, dietary intake of food forms of nutrients is not necessarily equivalent to supplementation with synthetic forms.
Food-form folate vs synthetic folic acid
The pattern that is beginning to emerge looks something like this: high-dose, synthetic folic acid can, in certain circumstances that are not altogether clear, decrease or increase cancer incidence. Another strand of evidence is just as relevant, however. When folate intakes are relatively high, and the folate comes from food, we see a more consistent and stronger picture of cancer-protective effects, with none of the apparent risks associated with synthetic folic acid.
Such relationships between natural and synthetic nutrients are, of course, not unfamiliar. With beta-carotene and vitamin E, for example, the natural forms have also been found to be superior, unburdened with the potential risks of their synthetic counterparts.
In the case of folate, synthetic folic acid represents its most oxidised state. If we consume this form, it needs to be converted to the forms usable by the body through the action of enzymes. The relative amount of the active form, notably 5’-methyltetrahydrofolate (5-MTHF), and the synthetic form is down to such factors as how much you take, how quickly it gets converted and how much is being used. This is a very variable and unpredictable process, and there’s a further complication: many people have defects in specific genes that are responsible for the enzymes required to convert folate into its primary active form, 5-MTHF. Among the many enzymes involved are dihydrofolate reductase (DHFR) and methylenetetrahydrofolate reductase (MTHFR).
There’s also an increasing suggestion that an excess of unmetabolised folic acid (UMFA) knocking around in the bloodstream may be central to the potentially harmful effects of high-dose synthetic folic acid supplementation.
Optimising your folate status
Distilling this complex scientific picture into some useful ‘take homes’ is one of the things we like to do at ANH.
Below is a 5-point strategy that you can adopt, with the help of a good nutritional or functional medicine practitioner, to help ensure your optimal folate status:
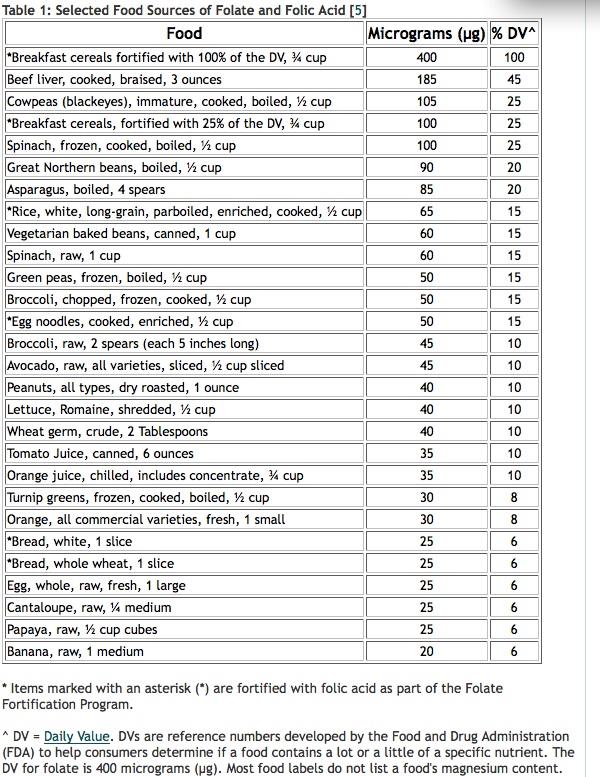
- Food forms of folate. Dietary folate, as found in dark-green-leaved vegetables such as spinach and kale, asparagus, green beans, peas, oranges and nuts, are good sources of folate and you want plenty of it – say 500–1500 mcg – daily. If you’re keen to check the dietary folate levels of different foods and how much you might need to eat to hit your target, which might be 1000 mcg/day or even more, you can use the USDA National Nutrient Database. One way to increase intake of natural folate is to juice green vegetables like spinach and French beans, which are rich in folate. Cooking can destroy up to 90% of naturally occurring folate
- Test your folate status. There are two main ways you can look at folate status: via red blood cells or in plasma/serum (blood). The former gives you a better picture of long-term status, and it’s something a good nutritional or functional medicine practitioner can help you with
- Supplemental forms. Not everyone can get enough folate from their diet alone. If you’re taking supplements, there are two European Union (EU)-legal forms of the stabilised, bioactive 5-MTHF available on the market. However, they are still available only in a small number of products – so check labels carefully. These forms are calcium L-methylfolate, often sold under the trade name Metafolin® produced by Merck, and Italian specialist Gnosis’ glucosamine-bound form, Quatrefolic®. There are also some products that contain other food-form sources, including 5-formyl tetrahydrofolate (5-FTHF), known also as folinic acid. In its synthetic form, this form is not allowed in food supplements in the EU, as it is not included on the positive list and is already registered as a drug, as a calcium salt, to help protect against some of the adverse effects of certain chemotherapeutic agents (trimetrexate, trimethoprim and pyrimethamine)
- UMFA testing. As an adult, if you’re taking folic acid supplements, especially high-dose ones, you’re more likely to run the emerging risk of unmetabolised folic acid (UMFA) in your bloodstream. If you’re worried about whether you have a problem with UMFA, you can be tested for it by a nutritional or functional medicine practitioner
- Genetic testing. There are 9 genes and 15 different enzymes involved in the staged reduction of folic acid to the active 5-MTHF form. Single-nucleotide polymorphisms (SNPs), i.e. defects, in the key enzymes are still being mapped and researched and there will inevitably be complex interactions between these polymorphisms. The relevance of these enzyme defects to our health will likely take many years to elucidate. But there are two key SNPs in MTHFR, at the 677 and 1298 positions, that can now readily be tested for. This is something a good nutritional or functional medicine practitioner can arrange for you – and it’s a good start for anyone whose folate deficiency persists despite apparent high intakes.








Comments
your voice counts
13 February 2014 at 1:42 am
A meaningless Q. Much like trying to apportion blame to synthetic beta carotene for causing cancer in the designed-to-fail Danish Study, when beta carotene neither causes nor prevents cancer. Dr H R Clark, PhD ND, doesn't even once metion folic acid in her latest 600 page 2007 work.
H R Clark, PhD ND, carried out over half a million reprpduceable, so scientifically valid bio-resonance tests to precisely identify the specific viruses, bacteria, parasites, dyes, heavy metals, moulds, radio-active compounds, benzene, asbestos, food allergens, etc that cause all cancers, and traced their pathways. Dr Clark needed to publish privately, so her work must remain 'unrecognised' and illegal for the doc to even mention. That also means that any cancer study today will and does ignore her work!
17 February 2014 at 9:00 am
Tank you for that information, which shows one more time, that it is best to eat as natural as possible.
Referring to the supplements:
I do not try any of those firms producing chemical extracts.
Why not supplement natural?
So I am missing the hint to use JuicePlus Premium Capsules as a supplement. (produced by NSA the JuicePlus company)
It is dried fruit and vegetable.
Best wishes
Dieter Klotzsche
17 April 2014 at 3:43 pm
Food Folate and Methyl folates are polyglutamates, they cannot be absorbed through the digestive trac until they are converted to monoglutamates. Then they go to the cell where they are converted back to a polyglutmate through MTHFR.
I don't see how taking Methyl Folate bypasses anything because of this step.
08 May 2014 at 4:59 pm
Thanks for your comment, Anonymous - and good question! It's an emerging field, but what we know at this stage indicates that polyglutamyl folate forms found in food, including 5-MTHF, appear to act differently to the monoglutamyl form of folic acid once they cross the intestinal brush border. The brush border contains deconjugase enzymes that convert the polyglutamyl form to a monoglutamyl form via deconjugation. Unfortunately, we don't yet know why this differential effect occurs. We also know that anyone consuming high quantities of 5-MTHF doesn't end up with excess unmetabolised, oxidised folic acid in their bloodstream, as we would expect to see if the conversion capacity of the brush border had been overwhelmed.
25 July 2014 at 3:33 pm
Great article! Do you know why so much attention is being given to people with a MTHFR polymorphism to avoid folic acid due to the fears of increasing UNFA? If someone had polymorphisms affecting MTHFR enzyme, that would affect the conversion of of MTHF (5,10-Methylenetetrahydrofolate ) to 5-methyltetrahydrofolate . FA is converted (via reduction) to DHF (dihydrofolate) via hidydrofolate reductase, which does not involve the MTHFR enzyme. So I’m not sure why someone would be more concerned with a buildup of UMFA in people with MTHFR mutations vs. people with normally functioning MTHFR enzymes. If anything, wouldn’t there be a buildup of MTHF in the bloodstream of people with MTHFR polymorphisms?
Thanks so much!
31 July 2014 at 11:41 am
Hi Chris, thanks for getting in touch. The best reason for the focus on MTHFR is the fact it has been very well studied. We know that MTHFR controls the amount of available 5-MTHF, the bioactive form, via its conversion of its immediate precursor - which, as you rightly state, is 5,10-methylenetetrahydrofolate. Additionally, the varying impacts of its various polymorphisms are now fairly well established.
But there are about 15 enzymes involved in the breakdown of folic acid to 5-MTHFR. Control of trafficking of polyglutamyl and monoglutamyl folates between the intracellular and extracellular (e.g. bloodstream) environment is critical for management of cell division, energy, methylation and many other essential processes. The key concern with UMFA is less the presence of the slow MTHFR enzyme, but more the fact that folic acid is the form that can pass through the gut lumen into the bloodstream without any further metabolic/enzymatic conversion steps. Therefore, the fact that around 40% of Americans have excess UMFA in their bloodstream is not linked to a slow enzyme, but rather to large intakes of folic acid in supplements and fortified foods.
In terms of enzyme polymorphisms, there are, of course, polymorphisms in many of the other enzymes that might also affect folate trafficking and an individual’s 5-MTHF pool. Key to this is trafficking between THF and DHF, and enzymes responsible for these conversions are the subject of active research at present. DHF reductase polymorphisms, for example, are emerging as a significant issue in some individuals, e.g. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3078682. Hope this helps.
12 December 2014 at 6:56 am
I'm SO glad I came across your article. It confirms my suspicion that folic acid may not only be less beneficial than folate but also be harmful.
I think that one day folic acid will be seen as problematic, just as retinal palmitate (synthetic Vit. A) was swapped out for beta carotene.
25 January 2015 at 9:46 pm
Thank you so much for your response. I’ve been perusing the clinical literature on MTHFR polymorphisms (as I have homozygous polymorphism) and I have to say that I’m having a very difficult time making heads or tails out of it. My homocysteine levels are within reference range and traditional allopathic medicine says that there is no cause for concern. However friends alternative medicine groups are popping up that are characterizing people with the aforementioned polymorphism as having a serious disorder. They are attributing the polymorphism to a host of syndromes and diseases. As you know, the published clinical literature correlating the polymorphism with various diseases (schizophrenia, depression, cancer, blood clotting, etc.) is of limited scope and somewhat equivocal. Some of those trials seem to be underpowered, in my humble opinion, so I went to the jury is out. Still, 5-methyltetrahydrofolate is involved in many biochemical and physiologic functions in the body and if I have a significant reduction in that compound, it is not implausible that I would be symptomatic. Additionally, as you discussed, if I have high levels of unmetabolized folic acid in my bloodstream, that could competitively inhibit 5-methyltetrahydrofolate from interacting with receptor sites and enzymes. And I could see how since I have lower levels of 5-methyltetrahydrofolate then the normal population how having high levels of unmetabolized folic acid could cause some significant impairment. To complicate matters further, some of the alternative medicine discourse is popularizing every other polymorphism that can be tested for and they are coming up with very robust theories and treatment protocols and are codifying certain disorders such as under and over methylators; which could be real clinical considerations, but I need to see some established research by medical professionals before I buy into that completely. It seems to me that they are extrapolating from the known physiologic functioning of various endogenously produced compounds and are just assuming that a deficiency or excess in any one of those automatically translates into a symptomatic disorder.
Anyway, I was wondering if you could answer a couple of quick questions for me. First, do you think people with an MTHFR polymorphism should limit their intake of foods containing natural folates as they could conceivably build up in the bloodstream? Am I understanding you though, when you say that only unmetabolized FA can enter systemic circulation to competitively inhibit 5-methyltetrahydrofolate, or can DHF also build up systemically and inhibit 5-methyltetrahydrofolate? If DHF does not enter systemic circulation, couldn’t it inhibit hepatic 5-methyltetrahydrofolate functioning?
Finally, do you know how much 5-methyltetrahydrofolate is produced in humans without polymorphisms daily? I want to customize my daily dose of methylfolate and avoid suprapharmacolic dosing.
Thank you so much for your time.
30 January 2015 at 6:08 pm
Re your first question: Please recognise that there is a considerable amount of folate produced by healthy gastrointestinal microbiota. So dietary requirements will vary for a variety of reasons, including polymorphisms which may affect 15 or more enzymes involved in folate metabolism, with MTHFR being the most researched, but with others such as DHFR now being researched intensely. It is also important to recognise that dietary folates are typically around 45% as bioavailable than monoglutamyl folate (Am J Clin Nutr 2004;80:911– 8).
There is little risk of eating too much dietary folate from food, and in fact there is growing evidence of widespread deficiency in particular subpopulations as measured by circulating 5MTHF and THF/DHF. 5MTHF status is maintained by a complex system of trafficking which is enzyme controlled. If you have ‘slow’ enzymes (e.g. MHRFR, DHFR) you are more likely to face deficiency, especially when need is high (e.g. for energy, cell division, immune response, methylation, etc.).
It is, however, easy to overdo FA intake as this runs straight into the circulation and if it is not sufficiently quickly converted in the folate cycle to 5MTHF, via the THF pool, you run the risk of excess UMFA which may lead to oxidative stress and consequent issues which may include increased cancer risk.
People with the MTHFR 677T polymorphism tend to end up with part of the 5MTHF being converted to formyl folates. So even if your dietary intake of food form folates is high, this is unlikely to result in harm in the way that high intakes of the oxidised form i.e. folic (pteroylmonoglutamic acid) does.
It is also important to maintain adequate levels of B12 in order to ensure that methyl synthase is not impaired given that it is needed to demethylate MTHFR to yield free folate, which occurs via the conversion of homocysteine to methionine.
Re your second question: I don’t know of any work that has attempted to quantify total 5MTHF production. This is because the gut microbiota will produce a large quotient of it, but the total amount will vary according to the relative health of the microbiota, consumption of prebiotics, fibre, etc. Also endogenous function will vary according to physiological/metabolic needs and dietary intake. What is much better understood is the status as measured via the circulation. In this regard, it is the red blood cell (erythrocyte) folate level that is the most accurate indicator of long-term folate status. Therefore this might be the most relevant clinical measurement, and if low, can be raised by consuming greater amounts of foods naturally high in folate, or consumption of a food supplement containing reduced (5MTHF) folate (or both!)
The USDA Nutrient Database is a useful portal to check dietary folate status of different foods (http://ndb.nal.usda.gov/ndb/). Alternatively, you may wish to use the UK CoFIDS database (http://tna.europarchive.org/20110116113217/http://www.food.gov.uk/science/dietarysurveys/dietsurveys/).
05 November 2015 at 4:51 pm
Update
It appears that 5-formyl tetrahydrofolate (folinic acid) has to be converted to 5,10-Methylenetetrahydrofolate first and then to 5-methyltetrahydrofolate via MTHFR.
I am unsure what enzyme converts folinic acid to 5,10-Methylenetetrahydrofolate. Since MTHFR converts 5,10-Methylenetetrahydrofolate to 5-methyltetrahydrofolate, wouldn't there still be a bottle neck if someone, like me, has a homogenous MTHFR polymorphism?
Interestingly, I see that in one study, conversion of 5-formyl tetrahydrofolic acid to 5-methyltetrahydrofolic acid is unimpaired in people who are homozygous for the C677T MTHFR polymorphism.
So I'm still confused here. So it appears that folinic acid has some intrinsic activity as it is needed for purine synthesis. So, if dietary folate intake is inadequate, supplementation would be warrented, but it wouldn't likely be the best option for people with a MTHFR polymorphism due to the aforementioned, correct?
12 December 2015 at 1:11 am
I wonder if some studies over the years have been inconclusive because those with the MTHFR polymorphism have inadvertently been included. It seems that if they were not weeded out, the results would be skewed in any study assessing cancer risk in otherwise healthy people.
12 December 2015 at 1:26 am
One other thought. You mentioned the difference between beta carotene and vitamin E, and the same can be said about bioidentical progesterone vs. synthetic progestins. Progesterone is often lumped in with progestin as a cancer causing agent, especially by the State of California, but my understanding is that progesterone, even aside from its protective value as an antagonist to estrogen, is an anti-cancer hormone on its own, or at the very least there has never been conclusive proof that it's cancer-causing. Folic acid supplementation is just one more instance where hundreds of millions of human beings have been sold a synthetic as safe and effective when in fact it's harmful. Why the U.S. government continues to require supplementation with folic acid is a mystery and borders on criminal. Not only should it NOT be in our food supply, it should be banned altogether.
23 May 2016 at 3:52 pm
To ban folic acid is easy for you to say...I take it to counteract the side effects of methotrexate! A more intelligent person would say to be careful when taking certain supplements, not to ban it.
14 November 2016 at 10:03 pm
If you don't have gene defects, that's fine. The argument here is the difference between synthetic folic acid and natural folate. Natural folate is the better option because of issues that people may have with metabolism, which they may not be aware of, and which may leave them seriously folate-deficient despite taking folic acid daily.
26 March 2017 at 5:12 pm
what is considered to be high dose folic acid?
27 March 2017 at 11:24 am
The dose used in the rat study referred to in the article (http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0084635) was 10mg, which approximates 1.6–2 mg folic acid/day (4–5x RDA) in humans.
Warm Regards
Melissa Smith
11 April 2017 at 11:58 pm
get your folic acid from vegetable. Do not be poisoned by the vitamin supplement industrial complex
11 June 2017 at 3:29 pm
Czyli.jak sie bierze 5 mg zwykłego kwasu.foliowego to jak to przeliczyc na metylowany kwas foliowy
17 August 2017 at 5:16 pm
Hello
Thank you for your question. Did you mean 5mg? Compared to the EU NRV of 200mcg per day, which we acknowledge can often be too low, 5mg is significantly higher than the NRV.
It isn't as easy as doing a straight mathematical calculation. Every person is different with different requirements. You should consult your healthcare practitioner so you can find out what your folate levels are and then decide in conjunction with your healthcare practitioner what, if any, action is required.
People with SNPs (genetic copying errors) can often need more folate and the methylated form is always best. Your healthcare provider will be able to advise accordingly.
We hope this helps.
Warm Regards
Melissa Smith
14 June 2017 at 10:03 pm
Nobody in the reseach community seems to disagree that supplementation is essential for pregnant women. The positive effects of folic acid supplementation would seem to outweigh the unproven risks. The benefits are proven, the risks are not. I doubt if 400mcg of folic acid is going to kill anyone so I will continue to take it with B12.
12 February 2018 at 4:46 pm
Folic acid is going to kill you slowly; digestive symptoms of gluten intolerance, pancreatitis; brain neurotoxicity poisoning with symptoms of insomnia, irritability, anger, depression, psychosis, cognitive impairment, memory loss, irrational behavior, changes in personality, and suicide; if one survives, the cancers so far clinically linked are lung, prostate, colorectal, and don't yet know.
WHO most common cause of deaths are #1 lung, #2 liver, #3 colorectal, #4 stomach, #5 breast
US most common cancers are #1 breast, #2 lung, #3 prostate, #4 colorectal, #5 melanoma, #6 bladder
Your voice counts
We welcome your comments and are very interested in your point of view, but we ask that you keep them relevant to the article, that they be civil and without commercial links. All comments are moderated prior to being published. We reserve the right to edit or not publish comments that we consider abusive or offensive.
There is extra content here from a third party provider. You will be unable to see this content unless you agree to allow Content Cookies. Cookie Preferences