Broken regulations affecting herbal products in the European Union (EU) and herbal practitioners in the UK are in the news again. The European Medicines Agency (EMA) has just published its latest, damning ‘school report’ on the EU’s Traditional Herbal Medicinal Products Directive (THMPD). Coupled with the stalling of UK government plans to bring in statutory regulation of herbalists, the future for herbalism remains manifestly unsettled.
New traditional use registrations down year-on-year
The latest EMA report on the THMPD covers the period up to and including 31st December 2012, in addition to the data in its two previous reports. A total of 264 new traditional use registrations (TURs) were granted EU-wide to herbal medicinal products in 2012 (Figure 1), bringing the grand total to 1015 (673 single-herb, 342 polyherbal) since the THMPD became law on 30th April 2004.
Of these 264 new TURs, 163 were for single-herb products and 101 for polyherbal products, the first time since the THMPD became operative that new TURs have dropped – by a whopping 29.6% – year-on-year. We appear to have passed ‘peak TUR’.
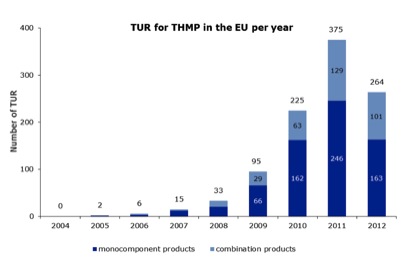
Figure 1. Number of TURs granted each year under the THMPD (2004 until 31st December 2012; total 1015).
Well-established use authorisations stable
Marketing authorisations granted under the well-established use (WEU) provisions of Directive 2001/83/EC have remained consistent (Figure 2), with 82 in total for 2012 – 59 for single-herb products, 23 for polyherbal formulations – up by 9 (12.3%) compared with 2011. Because of their increased rigour relative to the already strict THMPD, WEU marketing authorisations are only accessible to the largest and best-funded companies. And with the number of new TURs in steep decline, the smaller companies that are the lifeblood of the herbal industry are being shut out more than ever.
Not a very ‘traditional’ Directive
A closer look at the 342 polyherbal TUR products (Figure 3) shows that the vast majority (n=246; 71.9%) consist of 2–4 herbal components. There are 67 (19.5%) products containing 5–9 components, a drop in percentage terms compared with 2011 [/news/european-medicines-agency-releases-second-report-on-eu-herbal-directive]. In no way does this situation accurately reflect the practise of any long-standing herbal tradition, since formulae in the Western tradition commonly contain 5–10 herbs and those used by Indian Ayurveda and traditional Chinese medicine (TCM) often contain 12 or more. Ayurvedic chyawanprash may contain up to 80 herbs, and commonly includes 40–50 ingredients!
The incredible shrinking traditions
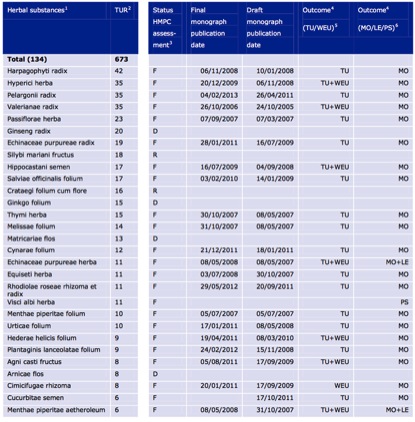
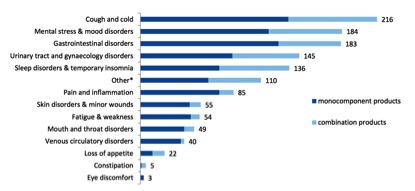
The pharmacopoeias of just Western herbal medicine, Ayurveda and TCM include up to 1,500 different plant species. By contrast, after nearly a decade of operation, the THMPD’s simplified registration scheme has allowed a total of 133 herbal species through its doors (Table 2), very few of which are associated exclusively with non-European traditions. The list of approved indications for TUR herbal products is similarly brief (Figure 4), consisting of a mere 14 therapeutic areas.
The THMPD: Not fit for purpose!
There’s no need for anyone to make a case against the THMPD based on the text of the legislation and its theoretical impact on the herbal products market, because the EMA’s own figures utterly condemn the herbal Directive. There is almost zero chances that common sense will prevail and EU regulators, supported by Member States, will see sense and repeal the law. The only practical way of fixing the THMPD, we believe, is through a favourable judgement at the European Court of Justice – which is what our legal challenge is all about.
UK herbalists in the long grass
Yesterday, on Tuesday 9th July, UK Member of Parliament (MP) David Tredinnick hosted a debate on statutory regulation (SR) of the country’s herbalists. Many people in the UK are wondering what their government is up to, after it promised SR in 2011 and then did precisely nothing. The Minister responsible for herbal medicine policy, Dr Dan Poulter, attended the debate, and his comments provided the first developments in quite some time.
In short, the government is kicking the entire issue into the long grass using the time-honoured politician’s technique of the infinite consultation. After literally decades of meetings, consultations, working groups, committees and focus groups to get us to this point, Dr Poulter proposed...another working group to discuss the “complex” issues raised by SR.
Polish problems
Once again, Dr Poulter is using as justification a recent judgement on a Polish case in the European Court of Justice (ECJ) that reveals Poland broke the law by importing cheap, unlicensed medicines – even though the imports were identical in terms of active substance, dose and form. The ECJ reached this conclusion because the imported medicines had not been granted a marketing authorisation according to EU medicines law. Dr Poulter’s comments on the Polish case confirm the statement made by Lord Pearson of Rannoch in a House of Lords debate on SR held on 24th April.
This is strange logic indeed, since SR would allow the UK to comply with EU medicines law whereas Poland was deliberately flouting it, albeit with some justification. But it’s heartening at least to know that the UK government thinks little of the argument proposed by skeptics like Lord Taverne, that SR would confer spurious respectability on a ‘quack’ profession.
We’ll be there!
Although we’re not convinced of the need for yet more discussions on this issue, we can confirm that we will be directly involved in them and we’ll keep you posted on developments. Along with others involved in the recent SR campaign, such as the European Herbal & Traditional Medicine Practitioners Association, we have been working hard behind the scenes to get to this point – so it’s a shame that none of these organisations were mentioned during yesterday’s debate.
EU herbal medicines legislation, then: it’s a complete mess. The sooner we can get into court and sort it out, the better – we look forward to your support!
ANH THMPD Legal Challenge page
ANH Nurture Traditional Medicinal Cultures campaign page








Comments
your voice counts
09 October 2013 at 2:59 pm
I'd be interested to know how you square two of your statements in this article:
No. 1) "In short, the government is kicking the entire issue (i.e. SR) into the long grass using the time-honoured politician’s technique of the infinite consultation"
and
No. 2) "Along with others involved in the recent SR campaign, such as the European Herbal & Traditional Medicine Practitioners Association, we have been working hard behind the scenes to get to this point"
as 1) and 2) seem non-congruent to me: "this point" is surely the point where SR has been kicked into the long grass, isn't it? I am sure that you did not mean that you wanted to arrive at such a point, but I'd be interested to try and understand the logic behind your points, and what it is you want to say.
Personally, whilst I support the ANH's stance on the THMPD, your stance on SR seems to me, from what I know of it, to be the wrong one. There are good reasons why the government could not statutorily regulate herbalists in line with the proposals of 2011, which would have ended up excluding large numbers of herbalists from practice, and some of us have been pointing this out for some time.
A less restrictive version of SR than that which appeared in the proposals of 2011 - one that would still allow all herbalists to continue to practise this most natural of therapies, with its track record of safety that is unsurpassed - would not, perhaps, meet with opposition from that many quarters.
10 October 2013 at 6:59 pm
Hi Phil, and thanks for your comment.
We don’t think there is any need to reconcile the two points: re-reading them, they seem quite clear to us and the points are quite separate. In the article, we say clearly: that the SR campaign, as the most recent manifestation of decades of work by herbalists’ groups, got us to the point where the government had promised to enshrine herbalists’ prescribing rights by enacting a statutory register of herbal practitioners. These proposals have now been delayed indefinitely by yet another government consultation.
As for SR itself, we detailed our position in two articles: http://anhinternational.org/news/stop-the-uk-government-doing-a-u-turn-on-herbal-medicine-promise and http://anhinternational.org/news/uk-herbalist-statutory-regulation-campaign-update. Our concern is for the prescribing rights of UK herbalists, which – as you’re no doubt aware – were until recently enshrined in the Article 12(1) exemption to the 1968 Medicines Act, and which now reside in Regulation 3(6) of the 2012 Human Medicines Regulations. SR would allow herbalists to be regarded by UK authorities as “authorised health-care professionals” under EU law (Directive 2001/83/EC), thereby allowing them to prescribe unlicensed herbal medicines for their patients, following a one-to-one consultation. The specific criteria governing their access to the herbalists’ register would determine how many currently practising herbalists would be excluded, and would represent a crucial campaign focus should the government ever again decide to go down the SR route.
However, the government is under significant pressure, from several sources as detailed in our articles, not to make herbalism a state-registered profession – and to repeal or reform the Section 12(1)/Reg 3(6) ‘herbalists’ exemption’. Should this worst-case scenario materialise, UK herbalists would lose their prescribing rights entirely; an unthinkable situation, but one that is all too realistic. Our position is this: the government has made a clear promise on SR and it should honour this promise; and SR, while not an ideal solution by any means, would give herbalists’ prescribing rights some degree of permanence. The latter point is especially important in an era where all forms of natural healthcare are under unprecedented legal and regulatory threat. Like you, we’d be pushing for the least restrictive version of SR; but, in contrast to what you alluded, we’re pretty sure that SR would still receive vigorous objection from some predictable quarters, irrespective of its permissiveness!
Of course, we would be even happier to see the maintenance of the herbalist’s exemption under Reg 3(6), i.e. the status quo, where there is no SR and herbalists maintain their existing prescribing rights. But, given the pressure being exerted on this, we’re worried that this provision won’t be maintained, so feel it is essential to have other options covered.
The reasons given by the government for not proceeding with SR have nothing to do with exclusion of herbalists from the register: it cites legal problems, including those following a recent Polish case involving import of unlicensed herbal medicines. To our knowledge, the government has not consulted with any herbal interests on the final shape of SR legislation since its announcement by then-Health Minister, Andrew Lansley MP, in February 2011.
11 October 2013 at 9:01 am
I hope you won’t object to my further analyzing what your position is, and then asking you for clarification(s).
You said: « the government is under significant pressure …. to repeal or reform the Section 12(1)/Reg 3(6) ‘herbalists’ exemption’. Should this worst-case scenario materialise, UK herbalists would lose their prescribing rights entirely; an unthinkable situation, but one that is all too realistic.”
- and then further on:
“Of course, we would be even happier to see the maintenance of the herbalist’s exemption under Reg 3(6), i.e. the status quo, where there is no SR and herbalists maintain their existing prescribing rights. But, given the pressure being exerted on this, we’re worried that this provision won’t be maintained…”
Could I ask what the factual basis is for your statement that the government is under significant pressure to repeal or reform the ‘herbalists’ exemption’? Considering that it has just been transferred virtually word-for-word into the Human Medicines Regulations 2012, your version could be considered as an exaggeration, as the government has just had every opportunity to repeal or reform it, but didn’t do so. So what, please, is the factual basis for your saying that you’re worried that this provision won’t be maintained?
The problem for your case is that this is precisely what we were told around twenty years ago by the pro-SR faction, that the government was going to scrap the herbalists’ exemption and we’d no longer have the right to practice, and so we had to have SR: but this turned out not to be true, and to have been just a form of scare-mongering by the pro-SR faction, in order to try to force acceptance of SR among herbalists.
Saying that you « would be even happier to see the maintenance of the status quo » does not, to me, square with your stated concern earlier on that « SR would allow herbalists to be regarded by UK authorities as “authorised health-care professionals” under EU law (Directive 2001/83/EC), thereby allowing them to prescribe unlicensed herbal medicines for their patients, following a one-to-one consultation.” Presumably, therefore, if this is as important as you say (which many would dispute it actually is, now that the situation is taking place), then you in fact want SR for this purpose alone, and not just because you have concerns about herbalists’ continued prescribing rights.
You seem to be crossing two threads of thought in saying that you “would be even happier to see the maintenance of the status quo”: yet surely you wouldn’t really be even happier, because you are calling for SR in connection with the EU Directive. To me, these two strands in your argument for SR do not square, and your position is therefore inconsistent. Your clarification would thus be appreciated.
11 October 2013 at 5:21 pm
We’ve no objection at all to an intelligent debate!
In the article we wrote announcing our SR campaign – http://anhinternational.org/news/stop-the-uk-government-doing-a-u-turn-on-herbal-medicine-promise – we asked:
“Why is a U-turn on the cards? [...]
“1. Pressure from the European Union (EU) institutions, in particular the European Commission and the London-based European Medicines Agency (EMA).
“The reform of EU medicinal law in 2011 granted “authorised health-care professionals” the ability to prescribe unregistered medicines for patients, an exemption principally designed to allow doctors to prescribe off-label. Ever since, there has been pressure on the UK to reform the herbalist’s exemption in British law that was won following intensive campaigns by herbalists back in the 1960s.” Neither the Commission nor the EMA look fondly at the herbalists’ exemption, as it goes against the principles of EU harmonisation by carving out a privileged status for UK herbalists. No other EU country confers a legal right upon herbalists to prescribe unlicensed medicines. Their regulatory authorities may be more lenient than the UK’s Medicines and Healthcare products Regulatory Agency (MHRA), but that’s another story. This quote also shows that the importance of “authorised health-care professional” status is that it maintains herbalists’ prescribing rights, a fact acknowledged by the MHRA in February 2011: http://www.mhra.gov.uk/NewsCentre/CON108789.
Furthermore, in Dr Verkerk’s open letter to Prime Minister David Cameron, he pointed out: “The MHRA and others have identified considerable weaknesses in this Regulation [Regulation 3(6) of the Human Medicines Regulations 2012], some of which we regard as valid.” The MHRA’s current position on Reg 3(6) can be found here: http://www.mhra.gov.uk/Howweregulate/Medicines/Herbalmedicinesregulation/Unlicensedherbalmedicinessuppliedbyapractitionerfollowingaonetooneconsultation/.
As we noted in February 2011 – http://anhinternational.org/news/yes-minister-or-not-contradictions-in-uk-government-policy-on-herbalist-statutory-regulation-0 – the MHRA certainly foresees the possibility of reform of what was Section 12(1), now Reg 3(6): “If practitioner regulation is in place for the purposes of creating an Article 5(1) scheme this also opens the way to reform Section 12 (1) of the Medicines Act 1968. Under Section 12 (1), practitioners may prepare unlicensed herbal medicines on their own premises for use following consultation with individual patients. It is intended to move to the position that only registered practitioners would be able to operate under Section 12 (1) after regulation of practitioners is in place.” The MHRA has also previously refused to confirm that it has rejected a move to a system of third-party commissioning of herbal prescriptions – which would end onsite prescribing.
The government is also under pressure on SR from the skeptic movement, which feels that making herbalists a state-registered profession provides respectability to a ‘quack’ practise. Fortunately, the government appears to be giving this nonsense short shrift.
To summarise our position again: the herbalists’ exemption is under threat; SR is not an ideal solution; but SR would legally protect herbalists’ prescribing rights under EU law. We’ll be delighted if the government comes out and states clearly that the herbalists' exemption is safe.
Finally, we’re not sure if you’re inferring that we are scaremongering, but either way, we would strongly recommend that you thoroughly read all of our published articles on SR, as we think our position is both clear and logically consistent.
11 October 2013 at 6:13 pm
Thanks for your response.
I'd asked what the factual basis was for your statement that "the government is under significant pressure to repeal or reform the ‘herbalists’ exemption' ", and you've said that "Neither the Commission nor the EMA look fondly at the herbalists’ exemption". This may well be the case, but is there anything more concrete than this that you can produce - documentation, minutes, reports, maybe - to support this latter statement?
25 October 2013 at 5:54 pm
Hi Phil, our full response in this regard was as follows: “Neither the Commission nor the EMA look fondly at the herbalists’ exemption, as it goes against the principles of EU harmonisation by carving out a privileged status for UK herbalists. No other EU country confers a legal right upon herbalists to prescribe unlicensed medicines.” This view has been developed from years of discussion, and as you’ll probably understand, even heated argument, with officials in the DG Enterprise, DG SANCO, EMA, the MHRA, MEPs and UK MPs and Lords. It seems that the uniqueness of the UK legal situation as regards herbal medicine practitioners (that are not accepted as being “authorised health-care professionals” under the terms of Article 5.1, Directive 2001/83/EC, by non-UK competent authorities or the EMA) puts it in direct conflict with the goals of EU harmonisation. We spend a lot of time lobbying and communicating with the key players driving these regulations, so rest assured that we are communicating the situation as accurately as we possibly can!
20 October 2013 at 4:24 pm
I have just returned from a fact-finding mission in the Iberian peninsula, to research the way in which THMPD and the health claims directive are being implemented in Portugal, the home country of the Lisbon treaty of which these directives form a part.
As long experience of dealing with governments has shown that, even the most simple and direct question, I have been met by responses drawn from the official collection of intentionally ambiguous platitudes, my research was conducted on the streets of Portugal, the home country of the treaty in question. I believe this research provided a functional “litmus test” of the current possibilities of interpretation and application of the EU directives under investigation. This is particularly important in the light of the proclaimed intention of the Under Secretary of State for Health, Dr Daniel Poulter, to investigate the possibilities of creating a national licensing system for herbal medicine products for sale in the UK.
1) Pre mixed formulas of cut herbal combinations are freely on sale in supermarkets throughout the country.
2) The packets, in which the cut herbal formulas are sold, carry a clear statement relating to their medicinal use.
3) Capsules of ground medicinal herbs (both singly and in combination) are sold as “Suplimento Alimentar” (dietary supplements).
These findings totally contradict the claims by the EHTPA that, without the imposition of statutory regulation, herbal medicine would no longer be freely available to the public, as the Portuguese precedent demonstrates this to be totally untrue.
Another factually aberrant statement, often put forward by the EHTPA, is that the practice of herbal medicine by herbalists is impossible by law on the continent. The fact that, until his recent return to the UK, one of the IRCH herbal medicine practitioners pursued a successful career in herbal medicine in Austria for many years is a direct repudiation of the EHTPA argument on this point as a justification for its pursuit of SR, under this argument.
The interpretation and implementation of the EU Health Claims Directive is clearly radically different in Portugal, forming a powerful precedent to challenge that of self-validating approach of the ASA in this country.
The licencing arrangements relating to natural herbal medicine products as dietary supplements also creates a powerful precedent to challenge the approach of pharmaceutical industry dominated MHRA in this country.
As evidence of these findings, I photographed the items in question on sale and purchased them, to bring back to this country as tangible proof.
I believe the above collapses the whole web of misinformation that has been peddled by the EHTPA and the financially interested parties it serves, to promote SR as the salvation of herbal medicine and its practitioners, when the exact opposite would inevitably be the case. The MHRA has declared its intention to remove the then section 12.1 of the 1968 Medicine Act, if SR came into being. This “right to practice” is currently still protected under section 3.20 of the 2012 Human Medicine Act, as quoted by the Under Sectary of State for Health, during the Early Day motion Debate in July of this year.
Robert Scott MIRCH (author Decoding Myth-Information)
25 October 2013 at 5:51 pm
Hi Robert, many thanks for your comment. First of all, we’d like to clarify that, “A national licensing system for herbal medicine products for sale in the UK,” already exists in the form of the Traditional Herbal Medicinal Products Directive (THMPD) and the corresponding Traditional Herbal Registration (THR) scheme. The UK Dept of Health is looking into the possibility of a nationwide statutory register for herbal medicine practitioners.
We applaud you on your proactivity in carrying out your own research in Portugal, but we’d caution you on the dangers of extrapolating your findings to the UK. What we think your research demonstrates is that simply having identical legislation in different EU countries is no guarantee that Member State competent authorities will interpret or enforce it in an identical manner. If EU law, in the form of medicines law and the THMPD, were followed to the letter in Portugal, the products you describe could not be sold legally unless they had been assessed and approved by the country’s equivalent of the UK’s Medicines and Healthcare products Regulatory Agency (MHRA). May we enquire whether the pre-mixed formulae you mention carried a) a registration mark/number, similar to the THR number in the UK, or b) a patient information leaflet? If both were present, the products are herbal medicines as defined by the THMPD, and are legal for sale just as THR products are in the UK. If not, and if they are not selling as food supplements – which seems unlikely, bearing in mind your third point – they are not strictly legal, and it may indicate that the competent authorities in Portugal are taking a more lenient approach to medicines/THMPD enforcement than the MHRA. We’ve certainly been aware of these variations for many years.
As for the herbal food supplements you mention, these so-called ‘botanicals’ are perfectly legal under the terms of the Food Supplements Directive (FSD). Many Member State competent authorities, including and especially the MHRA, are increasingly classifying botanicals as herbal medicines – meaning they have to jump through the THMPD’s narrow hoops or be removed from the market. You can find more detail on the problems with the FSD and botanicals in our information leaflets: http://anhinternational.org/sites/default/files/121030_ANH_A5_FSD.pdf and http://anhinternational.org/sites/default/files/121030_ANH_A5_Botanical_Ingredients.pdf.
It is also important to recognise that SR is a completely separate matter from herbal product legislation. We – and the EHTPA – are concerned that the UK government may reform medicines legislation such that herbalists lose their long-cherished prescribing rights. If that were to happen, herbalists in the UK could not legally prescribe unlicensed, individualised formulae to their patients. Whether these rights are maintained via SR or a continuance of the status quo is not important to us: what matters is that herbalists can continue to prescribe as they have for centuries, a right enshrined in law since the Medicines Act of 1968. We’ve outlined our position and reasoning on SR in detail in the conversation with Phil Evans, below.
It is correct to say that practising herbalism legally is challenging in most EU countries, if not impossible. The UK is the only country to include an ‘herbalist’s exemption’ for those that are neither doctors nor pharmacists, this exemption now being maintained in Regulation 3(6) of the UK’s 2012 Human Medicines Regulations. However, in reality, many – although by no means all – EU Member State governments take a more relaxed approach to interpreting EU law than the UK government and MHRA. The UK authorities are renowned ‘gold platers’ of EU legislation, often going beyond the letter of the law. By the same token, if the authorities in any Member State decided to take the same attitude as the MHRA and UK government, herbalists would soon find it very difficult to practise as they have become accustomed.
Finally, we’d be interested to see your evidence that Portugal’s attitude toward the Nutrition and Health Claims Regulation differs from the UK’s. This is particularly interesting because the NHCR is an EU Regulation and not a Directive, therefore it cannot be altered in its transposition into national laws. Only the interpretation and enforcement can be varied, although Member States may face challenge or fines from the European Commission for inadequate enforcement.
30 July 2014 at 1:58 pm
I am in contact now with my Green MEP who has asked for further information on this nonsensical legislation. Please can those of you with up to date information, please send to him any concise synopsis.
I am so seething after nearly being killed with gp script for cystitis, while in France and Hungary, over the counter herbs worked in a nanosecond. Gp comment "herbs don't touch it".
Further about a dozen herbs I regularly use from the USA mainly for reasons of economy, are now not available to uk buyers, or, are stopped at customs. Big PHARMA laughing all the way to the bank. Are we so powerless in a so called democracy.
KEITH TAYLOR GREEN MEP
www.keithtaylormep.org.uk
Email: [email protected]. [email protected]
Attn: Kathy Cadwallader
Your voice counts
We welcome your comments and are very interested in your point of view, but we ask that you keep them relevant to the article, that they be civil and without commercial links. All comments are moderated prior to being published. We reserve the right to edit or not publish comments that we consider abusive or offensive.
There is extra content here from a third party provider. You will be unable to see this content unless you agree to allow Content Cookies. Cookie Preferences